Extemporaneous Compounding
Introduction
In 2018, the Ministry of Health Malaysia published the Good Compounding Practice as a guidance for compounding practices. Upon reviewing the reference list of the guideline, you will come across the PIC/S guide.
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) was established in 1995.
- It aims at harmonizing inspection procedures worldwide by developing common standards in the field of GMP and by providing training opportunities to inspectors. It also aims at facilitating co-operation and networking between competent authorities, regional and international organizations, thus increasing mutual confidence.
- Malaysia became the 26th member of PIC/S in January 2022.
Preparation Methods of Oral Liquid
When preparing a formulation using tablets or capsules, it is necessary to thoroughly and uniformly pulverized them through trituration.
- Trituration involves reducing substances to fine particles in a mortar with a pestle. Small and uniform particles ensure easy dispersion throughout the vehicle or base solution, prevent quick settling and minimize the likelihood of caking. This is vital to ensure consistency and accuracy of dosing.
Once triturated, the powder should be levigated with a levigating agent.
- The levigating agent is selected on the basis of its ability to form a smooth paste with the powder to be levigated and on its compatibility with the substance.
The vehicle or base solution should be added to the paste in increasing amounts and mixed thoroughly.
- Geometric dilution = A small amount of the drug powder will be mixed into an equal amount of the diluent (e.g. into an ointment base). After the initial small is thoroughly mixed, another equal amount of the remaining ingredients is mixed. This is repeated until all the ingredients are mixed together.
The mixture should be transferred to a graduated cylinder.
- A small amount of vehicle or base solution should be used to rinse the mortar and the solution then poured into the graduated cylinder.
- The volume should be adjusted in the graduated cylinder to the quantity required for the formulation.
The final product should be transferred to a dispensing container.
NOTE: When compounding a preparation using the contents of an ampoule, care should be taken to withdraw the solution using a filter needle to avoid incorporating glass particles into the compound.
Mortars and Pestles
Mortars and pestles are used to grind substances into a finer consistency, and can be used to stir and mix small amounts of ingredients.
- Glass mortars are used for liquids, such as suspensions and solutions, and for mixing compounds that are oily or can stain.
- Wedgwood mortars have rougher surface than porcelain, and are preferred for grinding dry crystals and hard powders.
- Porcelain mortars have a smoother surface, and are preferred for blending powders and pulverizing gummy consistencies.
NOTE: During the dissolution phase, solutions should be stirred gently and uniformly to avoid air entrapment, which may result in foaming of the solution.
Measuring
In pharmacy practice, liquid preparations are normally made up to volume in a conical measure, rather than cylindrical measures.
Conical measures offer several advantages over cylindrical measures.
- They are easier to fill without spilling liquid on the sides above the required level.
- A conical measure has a wide mouth to make it easier to stir the mixture using a glass stirring rod. NOTE: The wider the mouth, the lower the measuring accuracy.
- It is easier to drain out the preparation.
- It is easier to rinse out the residue left after draining viscous liquids into the preparation.
- They are easier to clean after use.
On the hand, it must be borne in mind that compared to cylindrical measures, with conical measures
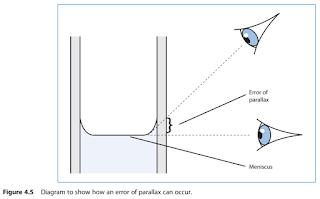
- It is harder to read the meniscus accurately.
- It is more difficult to estimate volumes between graduations (although in practice, owing to the error, this would be considered poor professional practice and is never done).
NOTE:
- Dissolution will normally not take place in a conical measure for a number of reasons.
- First, owing to the shape of the conical measure, any solid added will tend to "cake" at the bottom of the measure and hamper any attempt to stir the solid around with the stirring rod, which aids dissolution.
- Second, the action of the stirring rod may scratch the inside of the glass, permanently altering the internal volume of the measure.
- It is not a good practice to use a conical measure to measure a volume that is smaller than half of the total volume of the measure.
- The markings on a beaker are only approximations and cannot ever be used to measure any volume accurately.
- It is important to avoid spilling any liquid down the side of the measure as this could result in the incorrect amount of liquid being added to the preparation. It is also poor pharmaceutical practice.
Remember, when measuring liquids, that the bottom of the meniscus should be in line with the desired graduation mark. When reading the volume of the liquid, ensure that your eye is in line with the meniscus. This will avoid any parallax errors.
NOTE: Do not lift the measure to the level of your head as it will not be possible to read the volume accurately with the liquid moving about in the measure. Even if the liquid appears stationary, it is unlikely that the measure will be level.
There are occasions where a tared or calibrated bottle may be used. A tared bottle is normally only employed when, because of the viscosity of the final product, the transference loss from the measure to the container would be unacceptable.
- The volume of water added to the container to be tared must be identical to that of the product being prepared and must be accurately measured using a conical measure.
- When poured into the container, the meniscus is marked. (A simple method is to use a small adhesive label to mark the position and thus produce a measure with just one graduation.)
- The water is removed from the bottle and the bottle drained.
- The prepared mixture is transferred to the calibrated bottle, the measure or mortar used in the preparation of the product is rinsed with more vehicle and this is added to the bottle.
- Any liquid ingredients are added and the mixture is made up to volume using the vehicle.
- Remove the meniscus marker before dispensing the preparation to the patient.
NOTE: Graduations on dispensing bottles are not accurate and should not be used as a measuring device unless they are calibrated.
Weighing
With electronic balance, never place material to be weighed (e.g. powder) directly on the balance. The material will be placed on a weight boat (a shallow dish - made of plastic or other material) or on glassine weighing paper, which is coated to reduce moisture penetration.
- The compounder must "tare" or "zero out" the balance after placing the weigh boat or glassine paper on the scale.
NOTE: Equipment should be calibrated regularly to confirm accuracy.
Spatulas
Spatulas are used to mix and transfer (move) ingredients from one place (such as an ointment slab) to another place (such as a container). The flat part of the blade can be used to flatten and grind down ingredients, and to pack preparations such as ointments into containers.
Spatulas are made of stainless steel, plastic or hard rubber.
- A steel (metal) spatula would not be used if making a mixture that contains metallic ions.
- A rubber spatula is used to handle corrosive material.
Medicine Bottle
The following practices regarding medicine bottles were shared by a senior pharmacist assistant at my hospital.
- Different coloured caps are used to differentiate between internal and external preparations, with white caps for internal and red cap for external medications.
- Bottles with vertical ridges or grooves, known as fluted bottle, are used for external preparations. The vertical ridges serve as a tactile indicator for visually impaired patients (e.g. blind), allowing them to identify that the bottle is not meant for oral consumption.
NOTE: Medicines Act 1968 in the UK mandates that the use of fluted bottles for specific types of pharmaceutical preparations, such as embrocations, liniments, lotions, liquid antiseptics, other liquids or gels for external use.
Sorption to PVC Container
Regarding to clonazepam 0.1 mg/ml oral suspension, the X-temp Master Formulation Sheets, 2021 recommends the following:
- Packaging bottle: Amber glass bottle 100 ml; Avoid PVC containers; Shake well before use
- Storage: Room temperature, 25±2°C; Keep away from light
- Expiry: 60 days
While plastic bottle offers some advantages over glass bottle in terms of weight, fragility and cost, clonazepam solution exhibit loss due to sorption to PVC containers.
A 1996 study by Allen and Erickson evaluated the stability of three clonazepam 0.1-mg/mL oral suspensions extemporaneously compounded from tablets with three different vehicles and samples of each finished suspension were packaged in 120mL amber polyethylene terephthalate plastic prescription bottles and stored at 5 and 25°C in the dark.
- The study found no visual changes or changes in odour, and stability-indicating HPLC analysis found less than 5% clonazepam loss in any of the suspensions stored at either temperature after 60 days of storage.
Another study from 1990 investigated the sorption of clonazepam to polyvinyl chloride tubing, polyethylene-coated tubing and to a polyethylene syringe.
- The study observed a reduction in clonazepam concentration when pumped through PVC tubing, but no sorption occurred with polyethylene syringe or polyethylene-coated tubing.
Hence, it is still generally safe to use HPDE bottles for clonazepam 0.1 mg/ml oral suspension.
Safe Handling
Many hospitals prepare spironolactone syrups for their paediatric patients, especially when commercially manufactured spironolactone suspension is not available.
However, it is important to consider the hazardous nature of the spironolactone drug.
- Extemporaneous Preparations for Pediatric, Geriatric and Special Needs Patients, 2016 emphasized the need to prepare spironolactone suspension in compliance with USP 800 guidelines.
- According to NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016, spironolactone is listed in Group 2 because of the black box warning for tumorigenicity in laboratory studies.
NIOSH guidelines recommend double gloving, a protective gown, and preparation in a controlled device.
- If not prepared in a controlled device, respiratory and eye/face protection as well as ventilated engineering controls are recommended.
In my opinion, occupational safety, including how to handle hazardous drugs, is often overlooked in university education.
- The current curriculum primary focus has shifted from manufacturing to clinical aspects.
- While we are taught that we are the last line of defence for patients, but who safeguards us from potential dangers?
Furthermore, in late August 2020, the FDA approved labelling changes for hydrochlorothiazide (HCTZ) to inform clinicians and patients about the slight risk of developing non-melanoma skin cancer.
- This highlights that there is still so much we do not know about drugs and their potential risks.
External Links
- Pharmaceutical Compounding and Dispensing, 2010
- Pharmaceutical Society of Ireland Guidance for Pharmacists on Extemporaneous Dispensing, 2015
- Extemporaneous Preparations for Pediatric, Geriatric and Special Needs Patients, 2016
- Australian Prescriber Extemporaneously Compounded Medicines, 2017
- Pharmaceutical Society of New Zealand - General Guidance for Compounding Oral Liquids, 2019
- RxPrep's 2024 NAPLEX Course Book for Pharmacist Licensure Exam Preparation, 2023
- FDA approves label changes to hydrochlorothiazide to describe small risk of non-melanoma skin cancer, 2020



ReplyDeleteWow, what an insightful post! I particularly resonated with your point Your explanation clarified a lot for me. Thanks for sharing this valuable information. For more information this really helpful to your readers. Pvc Compound Manufacturer