Good laboratory practice (GLP) regulations – FDA requirements for laboratoriess
- Published on: Dec 24, 2023
The primary goal of pharmaceutical laboratories is to conduct sampling, testing, and reporting of medicinal batches.
As such, they perform a vital role in assuring the quality of medicinal products and, therefore, must conform to Good Laboratory Practice (GLP) regulations.
Several sections of the good laboratory practice regulations specifically relate to laboratories.
Government inspectors audit laboratory activities and records against these regulations, often focusing on adherence to GLP studies and the involvement of the quality assurance unit.
Several different laboratory regulations cover the lifecycle of pharmaceutical products.
For example, the term GLP describes the regulations concerning pre-clinical safety and toxicity studies.
The term Good (Control) Laboratory Practice G(C)LP describes the regulations and practices relating to pharmaceutical laboratory testing as part of Good Manufacturing Practice (GMP).
Due to their significance in pharmaceutical testing, this article will focus on GLP regulations for nonclinical laboratory settings. For the readers’ convenience, we will interchangeably use the terms G(C)LP and GLP.
Understanding GLP, G(C)LP and GCP
GLP: Good Laboratory Practices refer to the regulations concerning testing new drugs before conducting clinical trials. GLP rules are explicitly applied to toxicity testing of chemicals, drugs, and devices as a precondition for conducting clinical trials in humans.
G(C)LP: Good (Control) Laboratory Practices refer to the regulations concerning the specific laboratory testing of drug manufacturers under GMP.
In a sense, G(C)LP is a subset of GMP that specifically relates to the sampling, inspection, testing, and reporting test results. There is no separate set of rules; they are contained in the GMP rules themselves, which are in harmony with the principles of GLP studies.
GCP: Good Clinical Practices refers to the rules concerning the conduct of clinical trials in humans. This is a potentially dangerous phase of new product development, and the rights of the patients and volunteers must be protected. The GCP rules provide strict guidelines concerning Phase I, II, and III trials.
These rules are globally agreed to, but local differences remain.


240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Importance of good laboratory practices (GLP) regulations
Good Laboratory Practice regulations provide the framework that ensures the quality and integrity of non-clinical laboratory studies.
It sets the standards for performing tests and generating data in a reliable, reproducible, and valid way.
Compliance with GLP regulations is essential for pharmaceutical product research, development, and testing laboratories.
GLP is important because it helps minimize errors, enhance data integrity, and improve laboratory research quality in nonclinical studies.
GLP compliance also helps maintain laboratory personnel’s safety and the environment.
The history and development of GLP requirements
Good Laboratory Practice regulations have a rich history dating back to the 1970s.
Several incidents of fraudulent data and unsafe practices made the need for standardized practices and quality control in laboratory studies evident.
As a response, regulatory authorities in various countries developed guidelines and regulations to ensure the integrity and reliability of laboratory studies.
The Organization for Economic Cooperation and Development (OECD) was crucial in developing GLP. In 1981, the OECD published the first version of the Principles of Good Laboratory Practice, which provided a framework for implementing GLP.
Since then, many countries have adopted GLP requirements while aligning with the OECD principles.
Is GLP a legal requirement?
The Food and Drug Administration (FDA) and Pharmaceutical Inspection Co-operation Scheme (PIC/S) codes of GMP contain few specific references to Good (Control) Laboratory Practices G(C)LP.
However, they contain many indirect references within the codes and regulations directly applicable to the laboratory.
The points below outline some areas in the codes where GLP regulations are embedded in the GMP regulations and guidance documents.
1. FDA: 21 CFR regulations, especially those related to the conduct of nonclinical laboratory studies:
– Part 211, Section 160 (Laboratory Controls)
– Part 58 (GLP for Non-Clinical Studies) highlights the role of the study director in ensuring compliance.
– Guidance documents:
– Inspection of Pharmaceutical Quality Control Laboratories [1993]
– Analytical Method Validation & Chromatographic Methods
– Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production
2. EU and PIC/S: Guide to Good Manufacturing Practice for Medicinal Products:
– Section 2.6-27: Key Personnel
– Sections 6.5-6.6: Good Quality Control Laboratory Practice
– Annex 8: Sampling of starting and packaging materials
– Annex 11: Computerised systems
– Pharmaceutical Quality Control Laboratories
3. ISO/ IEC 17025: General requirements for the competence of calibration and testing laboratories. While not a mandatory laboratory standard, ISO 17025 provides excellent laboratory quality management systems guidance.
GMP, GLP and Quality Assurance in the laboratory
According to PIC/S, adherence to GLP in nonclinical studies is the only to ensure product safety.
Quality assurance (QA) is a broad concept covering all matters influencing product quality.
QA is the sum of organized arrangements to ensure that medicinal products are of the quality required for their intended use. QA “rules” incorporate GMP and GLP regulations and other factors.
Good Manufacturing Practice (GMP) refers to a set of licensing requirements to which companies must adhere to obtain and retain a manufacturer’s license.
The government and industry have agreed upon GMP rules, which have been gradually refined during 40 years of experience in practice.
According to PIC/S, GMP is that part of QA that ensures that products are consistently produced and controlled to an appropriate quality. GMP is concerned with both production and quality control.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
GLP compliance in analytical laboratories
GLP compliance encompasses many activities documented in the GMP and GLP rules. Four key aspects of GLP compliance are listed below.
1. Laboratory sampling
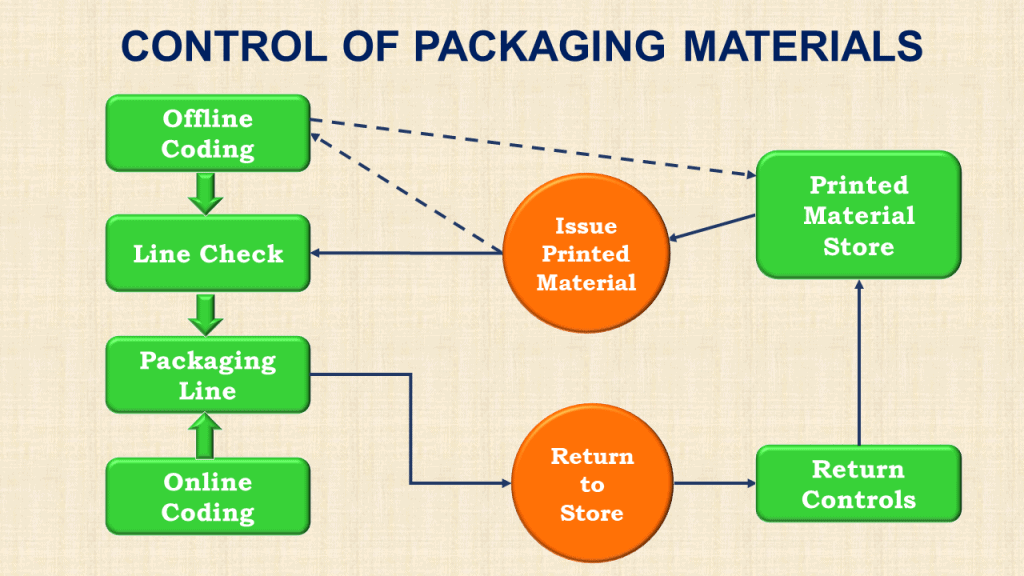
GLP regulations govern the sampling of starting materials and finished products. All sampling procedures and plans must be documented.
Any subsequent testing may give misleading results if wrong or insufficient samples are taken or a poor sampling technique is used.
As a result, a good product may be rejected, or, much worse, a defective product may be released.
2. Laboratory testing
Under GLP regulations, laboratory testing of samples is a mandatory requirement. However, its effectiveness is limited because the entire batch cannot be tested nor tested for all types of potential contamination.
QC testing is limited to looking for defects after they have occurred. So,it is not a QA prevention system but a defect detection system.
3. Reporting results
Laboratory documentation and records must follow the same rules as manufacturing documents.
To comply with Good Laboratory Practice regulations, the QC lab must have SOPs, test methods, specifications, registers, logs, and testing records in place.
These documents, such as the final report of a nonclinical study, must be current, approved, accurate, traceable, and archivable for later review.
Government auditors are particularly interested in the QC testing records when they conduct GMP audits.
4. Laboratory documentation
Each product has a specific set of specifications registered with the government authorities.
Starting materials and finished products must be tested to these specifications, and if there is a problem, the results must be reported to QA management.
Batches may not be released to the market if results do not conform to the approved specifications.
Some essential GLP requirements for laboratory operations
1. General GLP regulations
Laboratory activities and procedures must, in practice, conform to many Good laboratory practice regulations, which laboratory personnel should be familiar with.
These rules, particularly those related to the test article and test system, are commonly checked during regulatory audits.
In general, GLP regulations have a strong focus on:
– Conduct tests using approved, written test methods.
– Calibrate and quality instruments.
– All samples and standards are traceable and accounted for.
– Complete test records accurately and in real time.
– Validated methods support all tests.
– Record or capture all generated raw data directly, promptly, and legibly.
– Use traceable data sheets or sequentially numbered notebooks.
– Date and sign or initial data entries on the day of entry.
– Archive records so that they are protected, secure, and easily retrievable.
2. Regulations for laboratory instruments
Good Laboratory Practice regulations dictate that laboratory instruments must be reliable and clean to ensure proper performance and be qualified for use.
To ensure GLP compliance, you should take the following steps:
– Install all equipment and instruments according to the manufacturer’s instructions.
– Operate all equipment according to written procedures and relevant safety instructions.
– Keep all measuring devices within calibration.
– Regularly verify performance by checking controls.
– Implement a maintenance program and retain all maintenance documentation.
3. GLP compliance for laboratory documentation
GLP places a heavy emphasis on maintaining documentation. From a GLP compliance standpoint, it may not have happened if something wasn’t recorded.
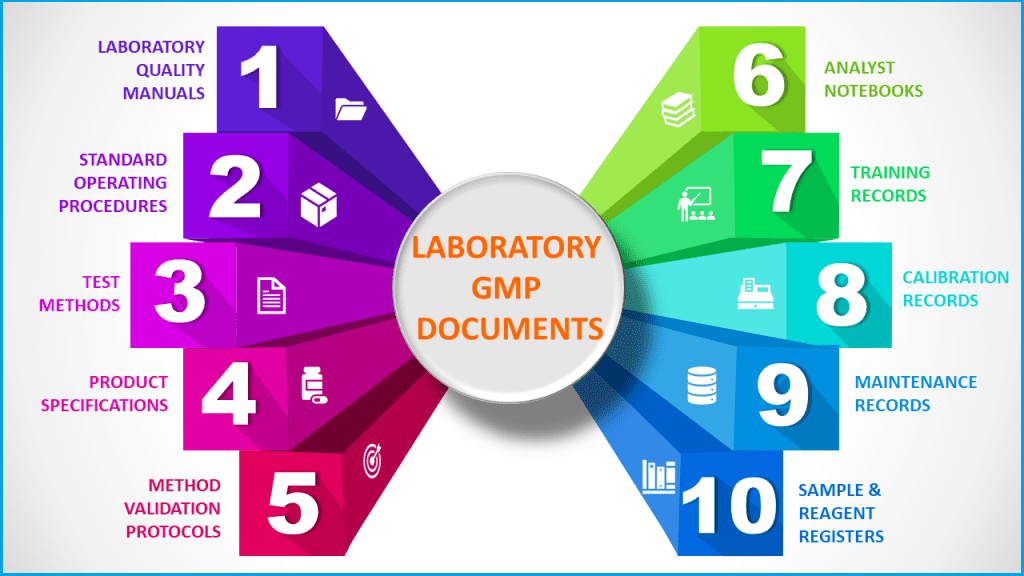
Laboratory documentation and records that should be available include:
– Test methods and test reports
– Specimen handling logs
– Laboratory notebooks/sheets, instrument records, and calculations
– Conditions of tests and instrument settings.
– Test method validation protocols, data, and reports
– Test control article records
– Other records and data
i. Testing and standardization of reference standards, reagents, and standard solutions
ii. Calibration of laboratory instruments
iii. Records of all stability testing performed.
iv. Investigations of Out-of-Specification (OOS) conditions in nonclinical laboratory.
v. Certificates of analysis from suppliers
Other examples of supporting records under GLP requirements include:
– Sample receipt registers
– Instrument and equipment maintenance logs
– Standards inventory lists
– GLP training records of the Analysts
– Retention sample storage lists
– Chromatograms and instrument print-offs
– Incubator temperature records (for a microbiology laboratory)
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
4. GLP training for laboratory personnel
The manufacture and testing of quality products rely on people.
Therefore, laboratory personnel must have the right skills, knowledge, and attitudes to perform their work correctly and diligently.
The types of GLP training required include induction training, on-the-job skills training, training on SOPs and test methods to understand laboratory procedures, and knowledge of GMP rules.
GLP training must be ongoing.
The company must demonstrate that training has been conducted satisfactorily in the above areas by monitoring each person’s training records.
Additional GLP regulations for personnel include:
– There must be sufficient laboratory staff.
– Personnel must undergo periodic health checks.
– Personnel must wear appropriate gowning.
– Personnel must always follow written procedures.
5. GLP on human errors and behavior
Many incidents leading to defective drug products result not from technological failures but from human error or simple mistakes.
Therefore, employees’ behavior, attitude, care, and knowledge are critical to GLP compliance and, eventually, the safety of medicinal products.
The laboratory must document procedures and quality control rules, but they have no value unless employees know and follow them in all their work practices.
6. GLP regulations of test methods
Laboratory test methods have a well-defined lifecycle from creation to publication to update and retirement. GLP regulations determine these pathways.
a. Source of methods: Test methods generally originate from one of the three resources. These include official pharmacopeias, new methods from a development laboratory, and methods adapted from literature.
b. Method development: During development, methods are tested for suitability according to the product they are being used for and the analytical validation performance parameters.
c. Published methods: Once a method has been suitably developed, it is published formally. The method should be unambiguous and easy for an analyst to follow, and it should also reference any instrumentation and corresponding settings.
d. Method validation: Once a method is published, it must be validated according to ICH Q2(R1) or equivalent criteria. This method validation package should confirm the method’s suitability. The validation report is generally submitted to a regulatory agency as proof that the method is fit for use and validated.
e. Method transfer to the laboratory: When a test method is suitably validated, it is transferred to the QC laboratory for commercial use. At this point, QC analysts become responsible for the test method.
f. Verify suitability: As part of the transfer, validated test methods must be verified as to their suitability in the QC laboratory. This generally involves partial validation to confirm that the transferred method remains in a validated state and is still fit for use in the QC laboratory.
g. Use method in testing: Once correctly validated, the test method is used for the routine testing of target products and should be reliable and robust.
h. Update methods: Test methods require occasional updates through change management. Some reasons for the update include alignment with pharmacopeias or improvements to the method. Updated methods generally require partial revalidation. The older version of the test method should be archived in case it needs to be referred to later.
7. GLP regulations of test method reliability
The following are the factors that support the GLP compliance of test methods:
– All test methods are validated to “fit for purpose.” Where required, they are revalidated.
– Methods are precisely written to minimize ambiguity and misinterpretation.
– Test methods are maintained in line with the current pharmacopeia.
– Laboratory analysts are trained and qualified in methods used in nonclinical studies.
– Laboratory equipment is calibrated and maintained.
– There are in-built performance checks per run (e.g., system suitability, controls, blanks)
– The laboratory undertakes trend reviews of assay performances.
8. GLP compliance for sampling plans and procedures
A written sampling protocol should exist for each starting material, in-process bulk, and finished product. Sampling plans should be based on sound statistical or rational principles and carried out in such a manner as to preclude bias.
The laboratory should publish written sampling procedures that describe the following:
– The method of sampling and environmental conditions
– The number, location, and amount of sample
– The sampling equipment
– Instructions as to the subdivision or proofing of the sample
– The identification procedure for sample containers
– The sample storage conditions
– Any safety precautions required
9. GLP requirements for internal auditing in the laboratory
Good internal auditing practices mean establishing a cooperative focus on “improvement” to the levels of compliance rather than issuing a list of non-conformances.
To do this, the audited department should be open and helpful to the audit group, and the audit group should be constructive in any criticisms.
After all, the purpose is to ensure that the laboratory meets all compliance standards. It’s in everyone’s interests.
Conducting audits as part of a quality assurance program is essential to any laboratory operating with a laboratory quality management system. ISO-17025 and regulatory agencies require this.
Audits are conducted to verify good procedures and practices and identify problems and improvement opportunities.
When conducting internal auditing, the laboratories should consider the following:
– GLP compliance audits
– Regular monitoring of test performances
– Quality system reviews
The credibility of the laboratory hinges on good quality management.
Documentation and records maintained in the laboratory should be able to withstand auditing. Specifically, internal audits aim to verify that:
– Results are reliable and accurate
– The information flow to customers is timely
– Test results are unambiguous
– The laboratory is economically efficient
– Historical records and data are retrievable
– The laboratory operates independently in quality assessment.
GLP regulations emphasize regular internal audits in the laboratory to ensure the trust of laboratory results. Be aware that loss of credibility results in:
– Loss of confidence and skepticism by users
– Inappropriate resource utilization, leading to excessive costs
– A focus on ‘defending” results
– Rejection of good and acceptance of bad quality
– An isolation of the laboratory
– Undue regulatory or external pressure on the company
Preparation for internal audits
GLP requirements dictate internal audits to be conducted in the following areas:
– Safety studies
– Compliance
– Regulatory
– Investigation
– Trend Analysis
Auditors will likely want to see laboratory notebooks, test methods, and “objective evidence” on how you conducted a test or calculated a result.
Have a list of all the documentation that might be needed and ensure they are readily available.
You may be asked because you are either an expert in the area or have particular knowledge of an issue or event.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
10. GLP regulations for external audits
External audits are generally conducted by purchasers, contractors, and accrediting bodies.
Generally, GLP regulations require external audits to include at least the following areas:
– The laboratory quality management system.
– The written procedures.
– The validation of test methods.
– The control over instrumentation and equipment.
– The test methods and how they are applied in nonclinical laboratory studies.
– The laboratory record-keeping practices.
– The procedures for evaluation and release of results.
Best practices for ensuring compliance with GLP regulations
Compliance with Good Laboratory Practice regulations requires a proactive and systematic approach. Here are some best practices to ensure GLP compliance in your laboratory:
1. Establish a culture of compliance
GLP compliance should be ingrained in the culture of your laboratory.
This starts with the commitment and support of top management. All personnel should understand the importance of GLP requirements and their individual responsibilities in achieving them.
2. Develop and implement laboratory procedures
Develop and implement robust procedures for all aspects of your non-clinical laboratory operations.
This includes SOPs for conducting experiments, performing tests, and documenting results. Procedures should be clear, concise, and consistent with GLP requirements.
3. Invest in GLP training and education
You can invest in training and education programs for your personnel.
Please ensure that all personnel have the necessary knowledge and skills to perform their tasks in compliance with GLP requirements.
Provide regular training updates to inform personnel about changes and updates in regulations.
4. Implement quality control measures
Could you implement quality control measures to monitor the reliability and validity of your data?
This includes regular checks of equipment, reagents, and test methods.
Establish a system for identifying and addressing any deviations or errors that may affect the quality of your studies.
5. Maintain accurate and complete documentation
Accurate and complete documentation is a fundamental GLP requirements.
Set up a documentation system that guarantees the traceability and integrity of all study-related data.
Train personnel on proper record-keeping practices and implement electronic systems for documentation and record-keeping.
6. Conduct internal audits
Conduct regular internal audits to ensure ongoing compliance with GLP regulations.
Internal audits help identify any non-compliance issues and provide an opportunity to correct them before external inspections.
Please ensure that internal auditors are independent of the nonclinical areas being audited and have the necessary expertise.
7. Prepare for external inspections
Prepare for external inspections by maintaining proper documentation and records. Be aware of the requirements and expectations of regulatory authorities in your country or region. Conduct mock inspections to assess your readiness and address any non-compliance issues.
8. Continuously improve and update
Good laboratory practice regulations for nonclinical studies are constantly evolving. Continuously improve and update your laboratory practices to stay in line with the latest developments in GLP regulations.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Conclusion
Pharmaceutical laboratories are integral to ensuring the quality of medicinal products through activities like testing and reporting batch results.
To maintain high standards, these laboratories must adhere to Good Laboratory Practice or GLP regulations.
Regulatory authorities such as FDA 21 CFR – Part 211, section 160 (Laboratory Controls) establish and maintain GLP regulations. The same can told for EU and PIC/S, Section 65-6.6 “Good Quality Control Laboratory Practice.
GLP requirements, a subset of Good Manufacturing Practice (GMP), specifically address daily laboratory testing activities. Another subset of GLP, Good Clinical Practices (GCP), regulates clinical trials, prioritizing patient safety.
Understanding the distinctions between GLP and GCP is important so you can focus on the specific regulations and requirements for your laboratory operations.
Compliance with GLP regulations is crucial for pharmaceutical research and testing laboratories conducting nonclinical laboratory studies.
It ensures the reliability of non-clinical studies, including medicinal safety studies, minimizes errors, enhances data integrity, and improves research quality.
Complying with GLP regulations also contributes to the safety of laboratory personnel and the environment.
Quick Takeaways
1. What is Good Laboratory Practice (GLP)
Good Laboratory Practice refers to the regulations for testing new drugs before clinical trials. GLP rules are explicitly applied to toxicity testing of chemicals, drugs, and devices as a precondition for conducting clinical trials in humans.
A subset of GLP regulations, Good (Control) Laboratory Practices, refers to regulations specifically focusing on drug manufacturers’ laboratory testing under GMP.
GLP regulations guide you on how to conduct sampling, inspection, testing, and reporting test results.
2. How to achieve GLP compliance?
To achieve GLP compliance, you should ensure that laboratory studies are planned, performed, monitored, recorded, reported, and archived to guarantee data reliability and ensure drug safety and quality.
Laboratory instruments are qualified and calibrated. Test methods are validated, and employees are adequately trained.
3. Why is compliance with GLP regulations important in laboratory studies?
Laboratory studies and testing must comply with GLP regulations, guaranteeing medicinal products’ safety, quality, and integrity. Failure to comply may result in deviation from standards, compromising product safety.
4. What are some key components of GLP regulations?
Some of the key components of GLP regulations include:
i. Development of standard operating procedures (SOPs),
ii. Well-designed sampling plan and method
iii. Preparation of test article
iv. Validated test or study protocols
v. Documentation and analysis of raw data
vi. Preparation of the final report that is unbiased.
5. How does GLP ensure the safety and reliability of laboratory studies?
GLP sets standards for conducting tests and producing data reliably, reproducibly, and validly.
GLP helps minimize errors, enhance data integrity, and improve the quality of laboratory research in nonclinical studies.
Additionally, GLP compliance contributes to maintaining the safety of laboratory personnel and the environment.
6. What are some of the best practices in GLP
Here are some best practices to ensure GLP compliance in your laboratory:
i. Establish a culture of compliance
ii. Develop and implement laboratory procedures
iii. Invest in GLP training and education
iv. Implement quality control measures
v. Maintain accurate and complete documentation
vi. Conduct internal audits
vii. Prepare for external inspections
viii. Continuously improve and update
7. Provide the names of regulatory bodies pioneering in GLP
i. FDA: 21 CFR regulations, especially Part 211, Section 160 (Laboratory Controls)
ii. EU and PIC/S: Guide to Good Manufacturing Practice for Medicinal Products.
iii. ISO/ IEC 17025: General requirements for the competence of calibration and testing laboratories.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
Process Validation Guidelines for Formulated Products
How to effectively control packaging materials in pharmaceutical
Types of Documentation Used in GMP Environments