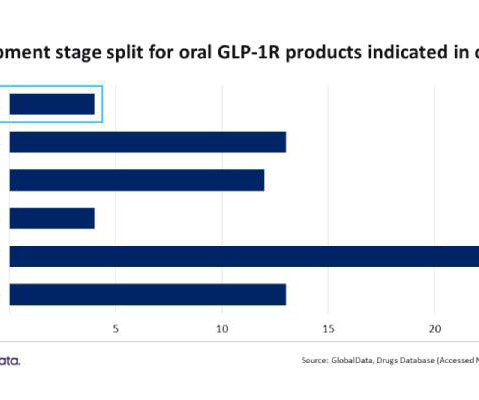
Race for approval of first oral GLP-1R drug in obesity intensifies with four in phase III trials: GlobalData
Express Pharma
DECEMBER 2, 2024
The company’s Phase III product Rybelsus, already FDA approved for cardiovascular risk factors and type 2 diabetes, is being positioned for a label expansion to include obesity. Novo Nordisk is leading the pack with four separate products, two apiece in Phase III and Phase II.























Let's personalize your content