Pregnancy in Patients with Epilepsy
Introduction
Epilepsy is a long-term condition and so people can be on medicines for a long time.
- Every year in the UK, around 2500 women with epilepsy have a baby.
- A 2014 study reported that many women with epilepsy report limited knowledge about key issues regarding pregnancy and childbirth.
Key Considerations of Epilepsy on Pregnancy
The structural and neurodevelopmental teratogenic effects of antiepileptic drug, especially sodium valproate.
- Evidence supports a rate of congenital malformations of 10% in infants whose mothers took valproate during pregnancy and neurodevelopmental disorders in approximately 30% to 40% of children.
Women with epilepsy are at increased risk for a range of perinatal complications compared with the general population, including preeclampsia, premature delivery, haemorrhage, foetal growth restriction, stillbirth and a dramatically increased risk of maternal mortality.
At least 50% of women with epilepsy will have no alteration of their seizure pattern during pregnancy, but some women experience seizure worsening compared with their baseline.
Pregnancy Planning
Clinicians should discuss the importance of planning a pregnancy for women with epilepsy who are of childbearing potential at each visit.
Such counselling should include information about
- Birth control and the potential of antiepileptic drugs to cause hormonal contraceptive failure
- The enzyme-inducing antiepileptic drugs are carbamazepine, oxcarbazepine, perampanel (at doses of 8 mg daily or more), phenobarbital, phenytoin, primidone and lamotrigine and topiramate.
- Antiepileptic drugs that do not affect hormonal contraception include clonazepam, ethosuximide, gabapentin, lacosamide, levetiracetam, pregabalin, sodium valproate, tiagabine, vigabatrin and zonisamide.
- The risks of antiepileptic drugs on pregnancy outcomes
- Possible changes needed to optimize the antiepileptic drugs regimen
- Importance of folic acid supplementation to prevent neural tube defects.
Principles for Treating Epilepsy in Females Planning a Pregnancy
- Is the diagnosis of epilepsy well established? Only continue treatment if needed to prevent seizures.
- If patient require antiepileptic drugs, is she on the most appropriate medication(s) and at the minimum dose to maintain seizure control? The risk of major congenital malformations associated with antiepileptic drugs is dose-dependent.
According to MHRA Public Assessment Report January 2021, lamotrigine and levetiracetam are the safer of the reviewed antiepileptic drugs during pregnancy because they are not linked with an increased risk of birth abnormalities compared with the general population.
- The available information also does not suggest an increased risk of the child having difficulties with learning or thinking ability but further data are needed to draw firm conclusions.
However, the clinician should weigh many factors when choosing which antiepileptic drugs to prescribe to provide the best balance between maternal seizure control and minimum side effects versus risks to the developing foetus.
- Prior medication failures (if any)
- Epilepsy syndrome and seizure type
- Seizure severity
- Adverse effects
- Comorbidities
Although it is especially difficult to change form an antiepileptic drug regimen that is working, some women will choose to change medications to an antiepileptic drug that has proven lower foetal risks while others will choose to stay on their current regimen.
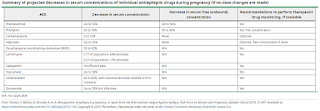
Antiepileptic Drug Adjustment and Monitoring During Pregnancy
Pregnancy is accompanied by many alterations in drug metabolism, including increased hepatic metabolism, renal clearance, and volume of distribution, as well as decreased gastrointestinal absorption and plasma protein binding. Hence, serum concentrations of some antiepileptic dugs may fall during pregnancy.
The 2019 ILAE report concluded that a decrease of more than 35% in the antiepileptic serum level (i.e. a fall to less than 65% of the optimal pre-pregnancy serum level) is associated with an increased risk of worsening seizure control. Hence, the goal is to adjust the antiepileptic dosing to maintain the specific target concentration(s) for each woman with epilepsy.
In the postpartum period, the rate of taper of antiepileptic drugs back to pre-pregnancy dose or slightly above depends mainly on the primary route of elimination for each individual antiepileptic drugs.
- The physiologic changes to renal and some hepatic enzymatic function (e.g., glucuronidation) associated with pregnancy will rapidly resolve over the first 2-3 weeks postpartum.
- Other hepatic enzymes (many of the CYP P450 enzymes) may take 1-2 months to return to baseline clearance rate.
External Links
- UpToDate - Management of epilepsy during preconception, pregnancy, and the postpartum period
- Management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy, 2019
- Antiepileptic drugs: review of safety of use during pregnancy, 2021
- New safety measure of sodium valproate, 2020
- National Patient Safety Alert: Valproate: organisations to prepare for new regulatory measures for oversight of prescribing to new patients and existing female patients (NatPSA/2023/013/MHRA), 2023

Very great post. I simply wanted to say that I have really enjoyed browsing your weblog posts. After all I’ll be subscribing on your feed and I am hoping you write again very soon!
ReplyDeleteEarthly Angels