From discovery to delivery: Fast-tracking patient access to life-saving rare disease treatments
pharmaphorum
FEBRUARY 28, 2025
From discovery to delivery: Fast-tracking patient access to life-saving rare disease treatments Mike.

pharmaphorum
FEBRUARY 28, 2025
From discovery to delivery: Fast-tracking patient access to life-saving rare disease treatments Mike.

FDA Law Blog: Biosimilars
FEBRUARY 23, 2025
By Steven J. Gonzalez & Allyson B. Mullen As the device industry is well aware, one of the greyest areas in device regulation (of which there are many) is determining when changes to a 510(k)-cleared device trigger the need for a new clearance. FDA requires a new 510(k) clearance when a modification to an existing 510(k)-cleared device (or other existing device subject to 510(k) requirements) could significantly affect the safety or effectiveness of the device.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Express Pharma
FEBRUARY 23, 2025
Chairman of Zydus, Pankaj Patel, emphasised the importance of people in organisational success during the launch of the School of Ultimate Leadership (SOUL) in Gujarat on Friday. Speaking at the event, formally inaugurated by Prime Minister Narendra Modi, Patel stated, People are the core to anything you do. The only mantra to succeed in any business is to take care of your people; other things will follow.
European Pharmaceutical Review
FEBRUARY 26, 2025
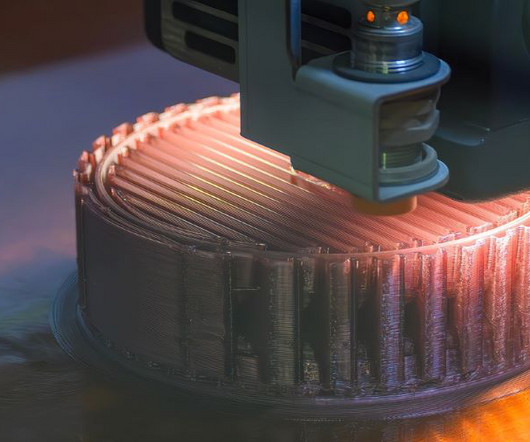
A study has demonstrated a new approach to prepare tablets for intestinal drug delivery by combining two 3D printing techniques. Using both selective laser sintering (SLS) and fused deposition modelling (FDM), the technique has potential to achieve delayed and prolonged drug release, according to the findings. Key findings from the 3D printing drug delivery study [a] laser speed of 90 mm s1 was suitable for producing [3D printed tablet] cores with relatively short disintegration time and suffici

Speaker: Jennifer Hill
Payroll compliance is a cornerstone of business success, yet for small and midsize businesses, it’s becoming increasingly challenging to navigate the ever-evolving landscape of federal, state, and local regulations. Mistakes can lead to costly penalties and operational disruptions, making it essential to adopt advanced solutions that ensure accuracy and efficiency.

PharmD Live
FEBRUARY 26, 2025
As specialty medications revolutionize treatment for chronic and complex conditions, their rising costs and intricate management requirements place a significant strain on patients, payers and providers. Without structured oversight, adherence issues, adverse drug interactions and financial barriers can lead to poor outcomes and unnecessary healthcare costs.

Pharma Marketing Network
FEBRUARY 27, 2025
Introduction In todays fast-paced world, pharma digital marketing is more than just a strategyit’s a necessity. With increasing competition, strict regulations, and changing consumer behavior, pharmaceutical brands must embrace digital transformation to stay relevant. But how can pharma marketers break through the noise, engage their audience, and see real ROI?
Pharmacist Digest brings together the best content for pharmacists from the widest variety of industry thought leaders.

The Pharmacist
FEBRUARY 26, 2025
Guidance for the education and training of pharmacists and pharmacist independent prescribers has been updated to reflect commitments to sustainability, the General Pharmaceutical Council (GPhC) has announced. The updated guidance highlights the crucial role of pharmacists as medicines experts in working towards the more environmentally sustainable use of medicines and decreasing their associated carbon footprint […] The post GPhC updates education and training guidance to support sustaina

Drug Patent Watch
FEBRUARY 27, 2025
Navigating the Complex World of Global Drug Patents: Strategies and Challenges Ahead As a pharmaceutical professional, you know how crucial it is to protect your innovative drug patents in the global market. With the rise of international trade and the increasing complexity of patent laws, it's more challenging than ever to safeguard your intellectual property.
European Pharmaceutical Review
FEBRUARY 27, 2025
Researchers at Pohang University of Science and Technology (POSTECH), South Korea, have developed a super-photostable organic dye. The discovery sets a new benchmark for organic fluorophores, Professor Young-Tae Chang explained, providing potential applications in broad areas such as drug development and cellular imaging. Single-molecule imaging uses fluorescent markers to track proteins with precision.

STAT
FEBRUARY 26, 2025
The U.S. Department of Agriculture announced Wednesday an additional $1 billion to help the nation’s poultry industry combat an accelerating outbreak of H5N1 avian influenza , which has devastated farmers and driven the price of eggs to record highs. The infusion is part of a new strategy under the Trump administration that aims to boost financial relief to farmers whose flocks have been affected by the bird flu and aid in increasing biosecurity measures to prevent the spread from w

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

The Pharmacist
FEBRUARY 25, 2025
Action on medicine shortages is vital to prevent another tragedy, the health and social care secretary has been urged. Charities and MPs have said the death of a man with epilepsy whose pharmacy was unable to supply his medication adds urgency to their calls for a review into the issue. David Crompton, aged 44, died […] The post 'Vital' health secretary intervenes over medicines shortages, urge MPs appeared first on The Pharmacist.

Express Pharma
FEBRUARY 26, 2025
Dr Reddys Laboratories has recently announced that it has received the Establishment Inspection Report (EIR) from the United States Food & Drug Administration (USFDA) for its Active Pharmaceutical Ingredient (API) manufacturing facility (CTO-2) in Bollaram, Hyderabad. The update follows the companys earlier intimation on November 19, 2024 regarding the USFDA inspection at the facility.

Pharmacy Joe
FEBRUARY 23, 2025
In this episode, I’ll discuss 3 types of ICU patients and why they matter for making patient-focused risk:benefit assessments. Episode 1003: The 3 Types of ICU Patients and Why They Matter for Making Patient Focused Risk:Benefit Assessments Subscribe on iTunes , Android , or Stitcher I find that I make better patient focused risk:benefit assessments when I start off by figuring out the answer to the following question: Why is my patient in the ICU?

STAT
FEBRUARY 27, 2025
Biotech venture capital is on the precipice of a generational shift. At least, that’s according to the crowd of younger firm founders edging their way in. There’s long been a small batch of scientists and business leaders who invest in drug companies. For the first 15 years of the 21st century, you’d find only 400, maybe 450 VC firms regularly giving money to drug startups — biotech’s own version of The Four Hundred , with lab coats or branded polar fleece, rat

Advertisement
Managing HR tasks like payroll, compliance, and employee data can overwhelm small businesses. That’s where a Human Capital Management (HCM) solution comes in. Our eBook, Why Every Small Business Needs an HCM Solution: A Comprehensive Guide , shows how an HCM system automates tedious processes, ensuring your business stays compliant and efficient. You’ll learn how to simplify payroll, eliminate costly errors, and empower your employees with self-service tools.

Drug Patent Watch
FEBRUARY 24, 2025
Breaking News: FDA Updates - What Do the Latest Changes Mean for Generic Drug Approval? Hey there, fellow healthcare professionals and industry experts! I'm excited to share with you the latest updates from the FDA that could significantly impact the generic drug approval process. As you may know, the FDA has been working tirelessly to improve the efficiency and transparency of the generic drug approval process.

Express Pharma
FEBRUARY 23, 2025
Lupin has announced that it has received the Establishment Inspection Report (EIR) from the United States Food and Drug Administration (US FDA) for its manufacturing facility in Somerset, New Jersey. The report follows an inspection conducted from 27 January to 31 January 2025. Nilesh Gupta, Managing Director of Lupin, stated, We are very pleased to have received the EIR for our Somerset facility.

The Pharmacist
FEBRUARY 27, 2025
Peers in the House of Lords have voted to exempt pharmacies from having to pay increased National Insurance contributions (NICs) from April. The amendment to the National Insurance Contributions (Secondary Class 1 Contributions) Bill says that specified employers, including those contracted to provide NHS pharmacy services, should only need to contribute 13.8%.
STAT
FEBRUARY 26, 2025
The Food and Drug Administration canceled an upcoming vaccine advisory committee meeting to discuss influenza virus strains, according to committee member Paul Offit. The meeting was scheduled for March 13. Committee members received a cancellation email — which was first reported by Jeremy Faust of Inside Medicine — on Wednesday afternoon.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.
Pharmaceutical Business Review
FEBRUARY 27, 2025
Carrying the Chinese trade name of Wan Ti Le, the tablets are indicated for individuals who have either insufficient response or cannot tolerate phosphorus binders. They are claimed to be the first to gain approval in the country, paving the way for multi-mechanism synergistic phosphate control while providing hope for individuals with hyperphosphatemia on haemodialysis in the country.

Express Pharma
FEBRUARY 23, 2025
The Appointments Committee of the Cabinet has approved the reappointment of Dr Rajeev Singh Raghuvanshi as Drugs Controller (India) at the Central Drugs Standard Control Organisation (CDSCO). The decision follows a proposal from the Department of Health and Family Welfare. According to an official notification issued by the Ministry of Personnel, Public Grievances and Pensions, Department of Personnel and Training, Dr Raghuvanshi will continue in his role on a contractual basis for one year from

The Pharmacist
FEBRUARY 27, 2025
A committee of influential MPs has called for an update on the highly anticipated economic review of community pharmacy in England. The Health and Social Care Committee (HSCC) has today asked NHS England to share when it anticipates the economic analysis of the community pharmacy sector will be fully completed, and if it intends to […] The post MPs ask NHS England if, when and how it will publish economic review of pharmacy appeared first on The Pharmacist.

STAT
FEBRUARY 27, 2025
In 2019, amid a measles outbreak in New York, federal health officials uniformly preached the power of immunizations. “Measles is preventable and the way to end this outbreak is to ensure that all children and adults who can get vaccinated, do get vaccinated,” said Robert Redfield , then the director of the Centers for Disease Control and Prevention.

Speaker: Joe Sharpe and James Carlson
Payroll optimization can be one of the most time-consuming and complex factors of small business management. Yet, organizations that crack the code on streamlining employee compensation often discover innovative avenues for growth. With the right strategies in place, outsourcing and streamlining payroll processes can result in substantial time and resource savings.

European Pharmaceutical Review
FEBRUARY 25, 2025
Scientists have developed a new, potentially safer method to produce ethylene oxide. Based on the proposed technique, adding small amounts of nickel atoms to silver catalysts can maintain production efficiency while eliminating the need for chlorine, leading to a reduced environmental impact for ethylene oxide manufacturing. What sparked this development?

Express Pharma
FEBRUARY 27, 2025
Global pharma company Lupin has been included in the S&P Global Sustainability Yearbook 2025 for the second consecutive year. The recognition is based on Lupins Corporate Sustainability Assessment (CSA) score for 2024, ranking it within the top 10 per cent of its industry. The S&P Global Sustainability Yearbook selects companies based on their CSA scores, evaluating their performance in sustainability.

The Pharmacist
FEBRUARY 27, 2025
There are MPs in Westminster advocating for community pharmacy funding, Dr Beccy Cooper MP told the sector at an event hosted by the Pharmacy Vaccinations Development Group this week. The event set out how community pharmacies could increase vaccination uptake and help the NHS reach those it had historically struggled to. And it accompanied the […] The post 'You have advocates in Westminster', MP tells community pharmacy appeared first on The Pharmacist.

STAT
FEBRUARY 27, 2025
The public and pharmaceutical companies are calling for changes in drug regulation and the costs of medicines. They are right to do so. Companies want to see products approved faster for financial reasons, and patients want medicines that can improve their lives. The two are inextricably tied, and realizing those goals requires tossing out the broken system we have in exchange for a superior system that is richer in data and evidence.

Advertisement
Running a healthcare facility requires precision and care, not just for patients but also for your staff. Our guide, "A Buyer’s Guide to Payroll & HCM Services," helps healthcare providers choose the best provider. Efficient payroll management ensures timely, accurate payments, critical for maintaining staff morale and trust. Compliance support helps navigate complex healthcare regulations and avoid costly fines.

FDA Law Blog: Biosimilars
FEBRUARY 26, 2025
By John W.M. Claud The ongoing DOGE-led reductions to the federal workforce and recent sweeping policy changes have spawned many questions for compliance officers and quality managers in FDA-regulated companies. How will the cuts at FDA impact inspections and enforcement? Will there be a heightened appetite for mergers and acquisitions in the space?

Express Pharma
FEBRUARY 27, 2025
Glenmark Pharmaceuticals Inc., USA (Glenmark) has launched Epinephrine Injection USP, 10 mg/10 mL (1 mg/mL) Multiple-Dose Vial. The product is bioequivalent and therapeutically equivalent to Epinephrine Injection USP, 10 mg/10 mL (1 mg/mL) of BPI Labs, LLC (NDA 205029). This launch qualifies for 180 days of Competitive Generic Therapy (CGT) exclusivity under section 505(j)(5)(B)(v) of the Federal Food, Drug, and Cosmetic Act (FD&C Act).

Drug Patent Watch
FEBRUARY 26, 2025
The Complex World of Biologic Drugs: Navigating Patent Applications As a biotech professional, you're likely no stranger to the intricacies of developing life-changing treatments. But have you ever stopped to think about the patent landscape surrounding biologic drugs? It's a complex world, to say the least. Biologic drugs, also known as biologics, are a type of medication made from living organisms or their components.

STAT
FEBRUARY 27, 2025
Federal health officials due to speak at the Healthcare Information and Management Systems Society meeting in Las Vegas next week have dropped out of the key health tech industry conference. Even as the Trump administration focuses on fraud, waste, and abuse, government officials aren’t allowed to tell the public about their programs aimed at preventing fraud, waste, and misuse in Medicare telehealth and remote patient monitoring programs.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.
Let's personalize your content