Drug Formulary
Introduction
Historically, a drug formulary developed by each and every hospital was to manage the proliferation of drug products.
- In just one year of 1951, the number of market entries consisted of 330 new drug products, including 35 new drug entities, 74 duplications of drug entities and 221 combination products.
- Hence, there would not be enough space for a hospital pharmacy to stock every single medicine that is marketed.
- To ensure that physicians had an adequate and consistent supply of medications for their day-to-day needs, the drug formulary system is being implemented.
Today, with pharmacy and therapeutic committee actively playing their role, this mechanism will discourage the use of marginally effective drugs and treatments.
- In other words, formulary system can be seen as an ongoing process that methodically evaluates medications on an ongoing basis for inclusion or exclusion; established guidelines for optimal medication use; and develops policies and procedures for prescribing, dispensing and administering medications
Access to Medicines
Formularies can be categorized by their access to medications as open or closed.
- An open formulary has no limitation to access to a medication. In other words, any prescriber from any discipline may prescribe this medication.
- On the other hands, a closed formulary, as the name suggested, is a limited list of medications that may limit drugs to specific physicians, patient care areas, or disease states via formulary restrictions.
However, formulary restrictions do not necessarily translate to optimal medication management.
- Hence, the key factor that we should carefully consider here is on the potential impact of formulary restrictions prior to implementation and to monitor actual impact after implementation.
- The formulary restrictions are justified on what basis?
Medication Selection and Review
Medication selection criteria should consider questions such as
- Is the medication under evaluation a duplication of an existing formulary drug? If so, what are the relative effectiveness, safety, tolerability and cost?
- How should it be used?
- When should it be used?
- Who should use it?
- Are there any other special concerns?
- For drugs that may be used upon patient discharge, are there patient access issues such as cost or availability in the community?
- How is the drug reimbursed? Is there a similar drug with better reimbursement when used in the outpatient setting?
Barriers to optimal formulary decisions may include
- Physician experience with the drug under consideration
- Physician preference for other agents
- Detailing by pharmaceutical company representatives
- Unpublished or anecdotal studies and report
NOTE: Critical elements are efficacy, safety and cost.
Therapeutic Equivalents and Therapeutic Interchange Programs
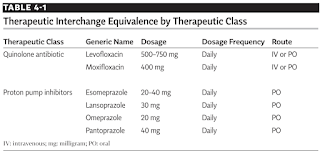
Therapeutic equivalents are drug products with different chemical structure but are of the same pharmacologic and/or therapeutic class and are expected to have similar therapeutic effects and adverse effects. Examples of therapeutic equivalents include quinolone antibiotics and proton pump inhibitors.
Therapeutic interchange is the authorized exchange of therapeutic alternatives in accordance with previously established and approved written guidelines.
Role in Inventory Control
The formulary is the cornerstone of the purchasing and inventory control system.
- The products on the hospital formulary dictate what the hospital pharmacy should purchase and keep in inventory.
- By narrowing down the range of medicines they keep in stock, pharmacy departments can negotiate larger discounts when purchasing and less likely to have medicines go out of date due to slow usage.
Creating Own Formulary Application
NICE have produced guidelines on developing and updating local formularies.
- Ideally, all relevant local formulary information should be published online in a clear, simple and transparent way to allow rapid adoption of new medicines and treatments, including formulary policies, minutes of meetings and decision outcomes.
My idea of creating drug formulary app was inspired by the Drug Formulary DIY app by developer Apicel.
- However, the app was unpublished from Google Play Store due to technical issue in the application coding.
- The developer had to rewrite the whole application from scratch, but he decided not to do so.
In 2020, I began to wonder if there were any IT professionals who had already made it easier for people like me to create mobile apps.
- I did a Google search and found an article about Glide, a tool that allows you to build mobile apps from a spreadsheet without writing any code.
Then, I decided to look into some alternatives.
- AppSheet - Perfect for personal use
- Caspio
- MDict
- Open as App - Useful for designing calculator type app too
- Wistify.app - Basic app with security password feature
NOTE: Only MDict does not offer real time database update.
Summary
Apart from a list of medicines, many drug formularies have other content as well, such as
- Advise about the preferred first choice medicine for a condition
- Specify who can prescribe certain medicines.
- Advise on drug administration and storage.


Comments
Post a Comment