Adverse Drug Reactions
Introduction
One common question that we encountered at work is if this drug has any adverse effects?
- Inherently, most drugs possess undesirable adverse effects, some of which may be unobserved or unknown.
- Therefore, clinical decision-making often involves balancing the potential benefits and harms of a treatment.
Adverse Drug Reactions
The World Health Organization (WHO) defines an adverse drug reaction (ADR) as 'a drug-related event that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function'.
However, this definition does not consider the following scenarios, all of which can also cause ADRs.
- Overdose (including prescribing or administration errors)
- Therapeutic failure
- Drug interactions
- Drug withdrawal
NOTE:
- All adverse reactions are adverse drug event, but that not all adverse drug events will be adverse drug reactions.
- Adverse effects can be difficult to differentiate from background events occurring commonly in the population.
Frequency
Although adverse drug reactions are listed in product leaflet, it does not necessarily occur to the patient.
- Rather, terms such as 'common' and 'uncommon' are often used in product leaflets to describe levels of risk of ADRs.
- To avoid misunderstanding of public on the level of risk, we should avoid using verbal descriptors such as 'common', but to use frequencies in patient counselling - e.g. 1 person in every 1000.
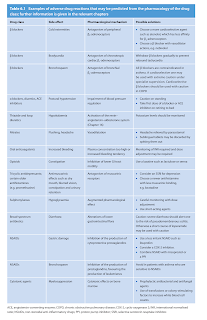
Classification of Adverse Drug Reactions
Adverse reactions can be classified as
- Type A, which are most common and related to the drug's pharmacological effect
- Are often dose dependent and may often be managed by dose reduction.
- If they are anticipated, measures may be taken to ameliorate or prevent the problem.
- Type B, which are rare and unpredictable,
although other classes of reaction can be identified.
In other words, there are adverse drug reactions that we can predict based on pharmacological action, but there are also unpredictable one.
In contrast with Rawlins-Thompson classification, the DoTS classification is based on dose relatedness, timing and patient susceptibility.
Factors that Affect Susceptibility
- Age - Elderly, neonates and children have an increased susceptibility
- Comorbidities, e.g. renal impairment and hepatic impairment
- Polypharmacy
- Female
- Pharmacogenetics
- Glucose-6-phosphatase dehydrogenase (G6PD) deficiency
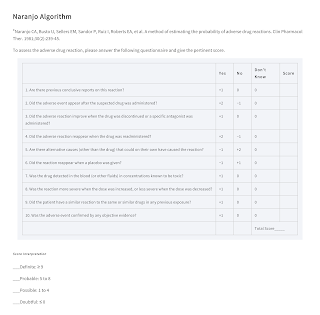
Causality Assessment
The TRIP acronym can be used to help determine whether an ADR has occurred.
- Timing
- Depending on the type of adverse reaction, its time to onset can vary from minutes after receiving a drug to months.
- Recovery
- Do symptoms improve or resolve completely when the suspected drug is stopped?
- Does the reaction improve when a specific treatment is given? (e.g. procyclidine to treat dystonia caused by metoclopramide.)
- Independent evidence
- Is there another plausible non-drug cause for the problem?
- A rechallenge will cause symptoms to recur if the suspect drug is restarted.
- Predictability
- Are the characteristics of the adverse event consistent or inconsistent with the known pharmacology of the drug?
- Check whether similar events have been reported previously with the drug or a related drug.
Also, several methods of assessing causality have been developed using standardised decision algorithms to increase objectivity and reduce assessor bias.
- One of the most commonly used is the Naranjo algorithm.
Managing ADRs
When confronted with an ADR, ask yourself:
- The severity of the reaction.
- Is this a dose-related reaction of not?
- Whether you are certain that you know which drug is responsible.
- The consequences if you stop the offending medicine. Is it safe? What other medicines can be used instead?
- Whether a dose reduction is feasible or practical.
- The ability to successfully treat the side effect.
Also, it is important to
- Document the ADR in the patient's medical record.
- Report the ADR to the appropriate authorities to help track the incidence of ADRs and identify any potential safety concerns.
- Educate the patient about the ADR.
- Monitor the patient for any further changes in their condition.
Reporting to the Appropriate Authorities
When drugs are newly introduced to the market, their safety profile is inevitably provisional (i.e. limited data available).
- Pre-marketing trials do not have the power to detect important reactions that occur at rates of 1 in 10,000 or fewer, drug exposures.
- Additionally, patients within trials are often relatively healthy, without the multiple disease states, multiple risk factors or complex drug histories of patients in whom the drug will be used.
Therefore, the vigilance of health professionals is an essential factor in discovering these new risks, together with regulatory authorities that continuously monitor reports of adverse effects throughout the lifetime of a marketed medicinal product.
- For Malaysia, information on reporting adverse drug reactions can be found here.
- In UK, it is reported through yellow card scheme.
NOTE: A major reason for drug products to get a "Black box" warning that may lead to market withdrawal is that studies conducted before the drug enters the market (premarket) fail to detect uncommon drug effects.
Summary
As pharmacists, we are obligated to inform patients about the potential adverse effects of newly prescribed medications, particularly those that are common or require immediate medical attention.- Furthermore, we must proactively advise patients on the management of these potential adverse effects.
- The initiation of a prescribing cascade, intended to mitigate adverse drug reactions, should be avoided unless the additional medication is absolutely essential.
External Links
- Malaysia: Adverse drug reactions vs side effects
- Malaysia: NPRA - Reporting ADR
- Malaysian Guidelines on Good Pharmacovigilance Practices (GVP) for Product Registration Holders, 2021
- WHO: Pharmacovigilance
- The assessment of severe cutaneous adverse drug reactions, 2022
- Medicines Learning Portal - Adverse Reactions
- Yellow Card - Specific Areas of Interest for Reporting Suspected Adverse Drug Reactions
- Pharmacovigilance in Australia: how do adverse event reports from clinicians contribute to medicine and vaccine safety?, 2025




Comments
Post a Comment