Validation master plan (VMP) – when and how to create one?
GMPSOP
MAY 31, 2024
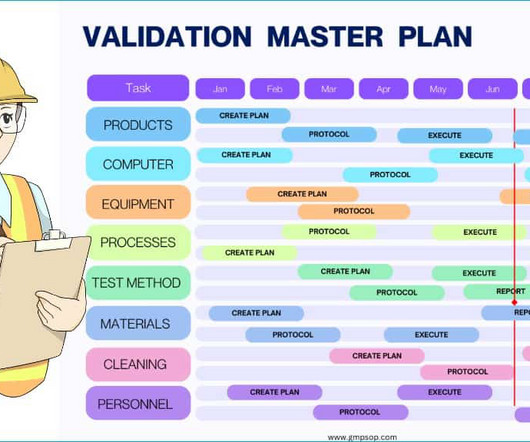
If you are in the pharmaceutical business, regulatory authorities mandate that you develop, implement, and periodically review a validation master plan for qualifying your systems, processes, cleaning method, testing methodologies, equipment, facilities, etc. Method validation master plan?











Let's personalize your content