Best CDMO Practices for Startups: Navigating the Complex World of Contract Development and Manufacturing
Drug Patent Watch
JANUARY 9, 2025
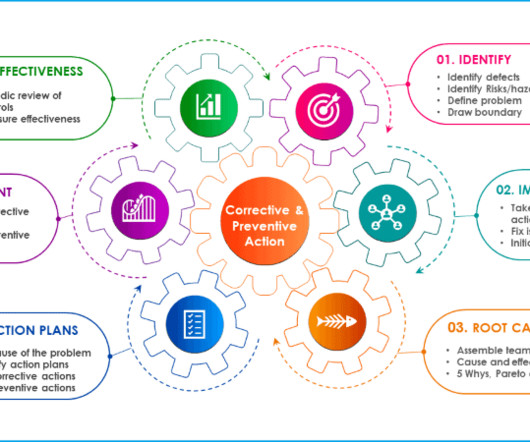
Take the time to clearly define your technical needs, from drug substance manufacturing to packaging requirements. The CDMO Selection Process: A Step-by-Step Guide Selecting the right CDMO is a critical decision that can have long-lasting impacts on your startup’s success.








Let's personalize your content