Effective Control of Microorganisms in Pharmaceutical Manufacturing
GMPSOP
JUNE 15, 2025
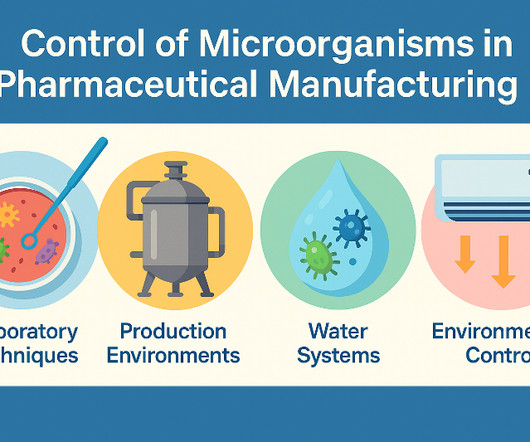
Effective Control of Microorganisms in Pharmaceutical Manufacturing Pharmaceuticals quality assurance & validation procedures GMPSOP %title% Prev PREVIOUS POST The control of microorganisms in pharmaceutical production ensures that they stay at acceptable levels. Additional documents are included each month.

















Let's personalize your content