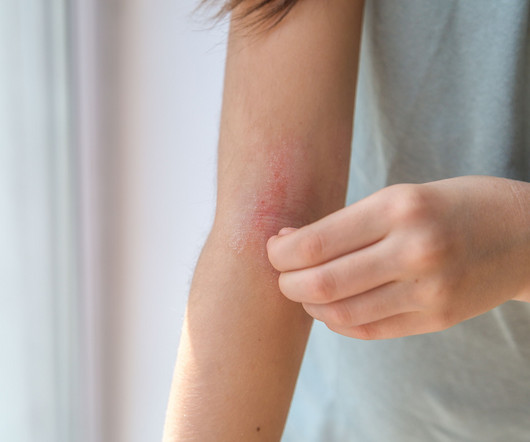
Nemolizumab Shows Rapid, Sustained Efficacy in Atopic Dermatitis
Pharmacy Times
JUNE 16, 2025
The findings emphasize nemolizumab’s swift response in treating atopic dermatitis symptoms, along with its long-term effectiveness in prurigo nodularis, as demonstrated in the OLYMPIA open-label extension trial (NCT04204616). Mayo Clinic. News release. June 6, 2025. Accessed June 16, 2025. Atopic Dermatitis. News release.













Let's personalize your content