Overview of pharmaceutical quality control steps and implementations
GMPSOP
JUNE 9, 2024
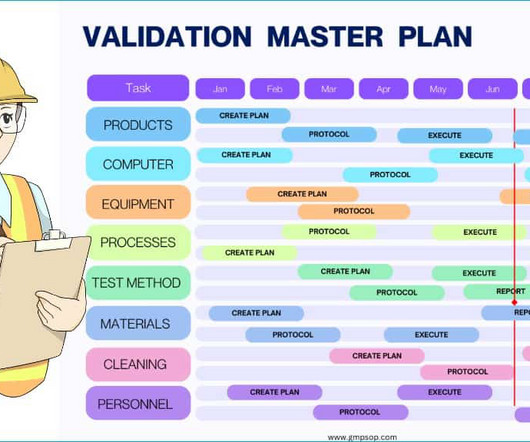
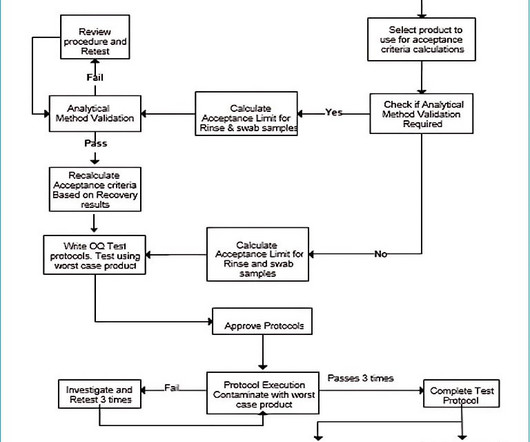
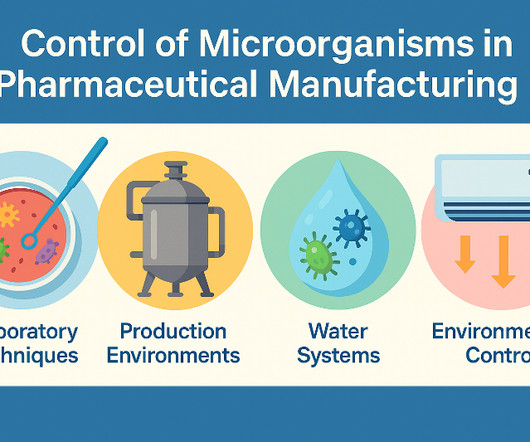
Test method validation in quality control Analytical Method Validation for Quality Control Test method validation generally means the same as analytical method validation, where the terms are interchangeable. Maintenance programs must be documented for laboratory equipment.









Let's personalize your content