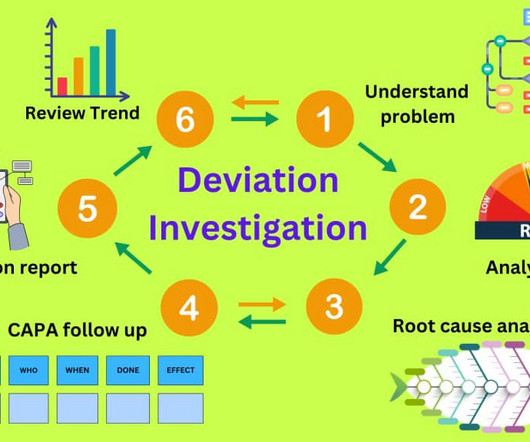
Deviation investigation guidelines in the pharmaceutical industry
GMPSOP
JUNE 28, 2024
Packaging/labeling/Misbranding: 41 warnings vi. Similarly, the packaging, labeling, and misbranding-related warning letters were the top reasons in 2019. incorrect data entry). This is a critical deviation since the medicinal batch does not comply with the label claim (strength) and violates regulatory expectations.







Let's personalize your content