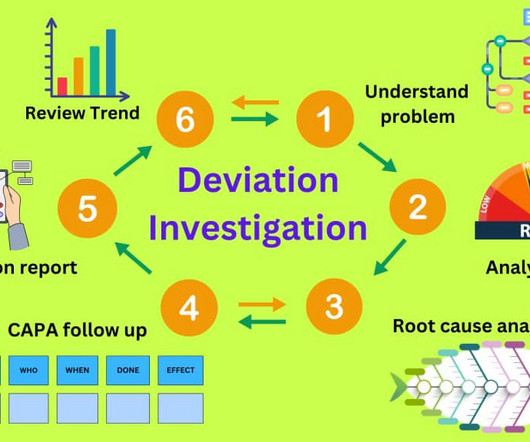
Deviation investigation guidelines in the pharmaceutical industry
GMPSOP
JUNE 28, 2024
It is important to understand that everyone in the pharmaceutical manufacturing facility is responsible for reporting a deviation as soon as one is identified. incorrect data entry). Example: An incorrectly spelled product name on the manufacturing instructions can be classified as a minor deviation.







Let's personalize your content