How to use quality risk management in validation testing
GMPSOP
AUGUST 23, 2022
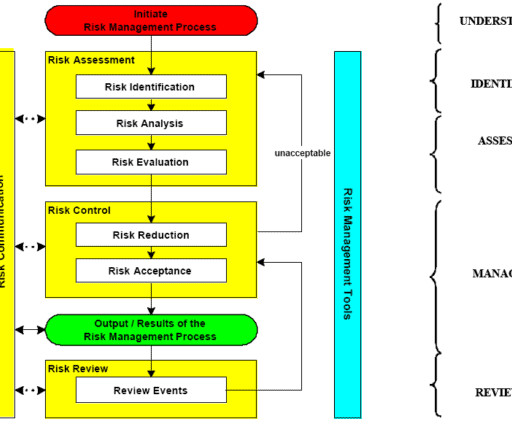
Table of Contents Quality risk management principles are already effectively utilized in many areas of pharmaceuticals manufacturing business. Risk management scope definition The purpose or objective of the application of risk management needs to be clearly understood. Subscribe Step 1.











Let's personalize your content