FOPE and PharmaState Academy hosts Session 11 of the PULSE series
Express Pharma
DECEMBER 23, 2024
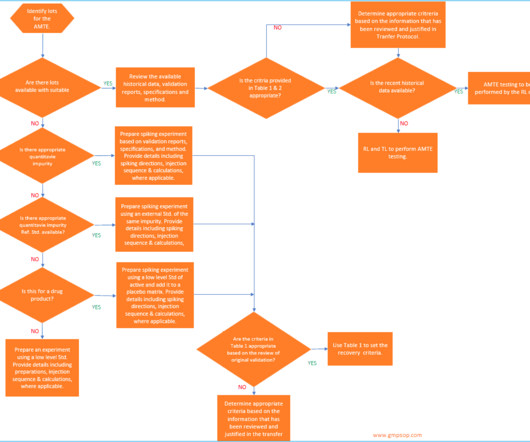
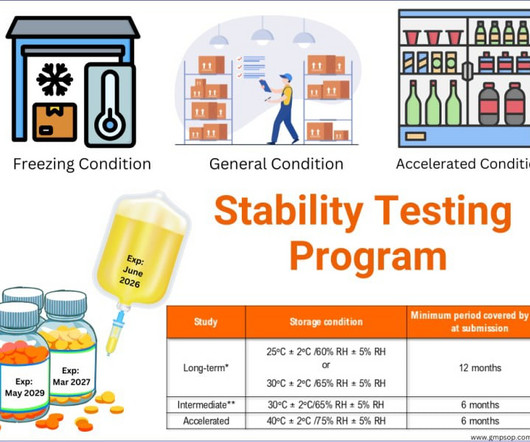
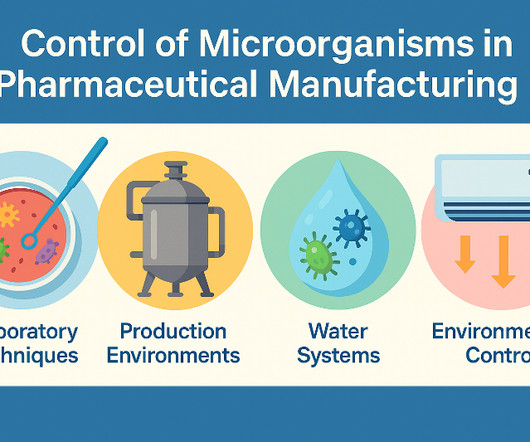
He also discussed the importance of having retention samples for raw materials and packaging materials and the role of quality control in investigating deviations and ensuring the stability of raw materials. He also stressed the importance of reviewing batch records and analytical records and the need for clear documentation practices.











Let's personalize your content