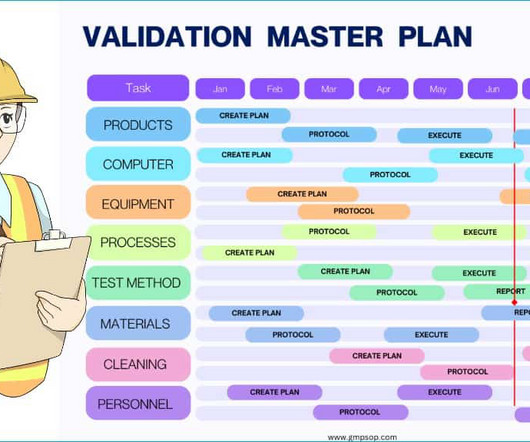
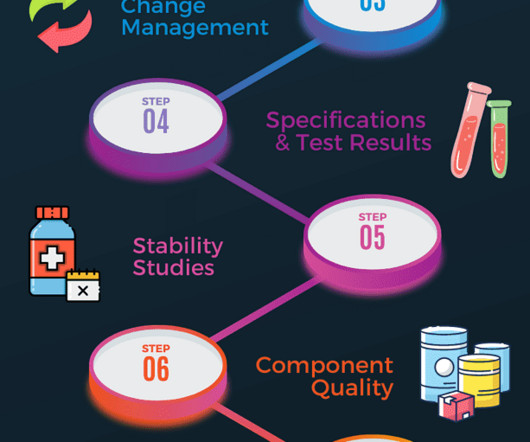
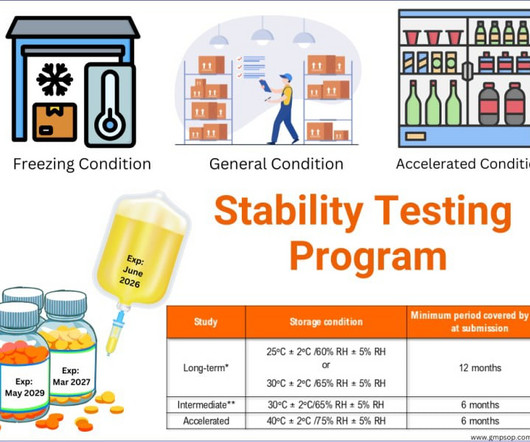
Concept of validation in pharmaceutical industry
GMPSOP
OCTOBER 26, 2022
Additionally, validation includes continuous monitoring and re-evaluation to ensure that the product or process remains in compliance with the established requirements. By implementing robust validation protocols, pharmaceutical companies can ensure that their products are safe, effective, pure and of the highest quality.










Let's personalize your content