Labelling of Dispensed Medicine
RX Note
DECEMBER 25, 2024
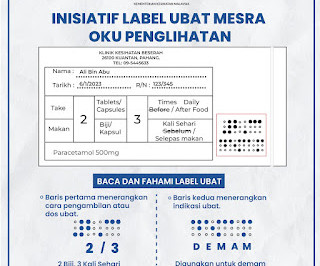
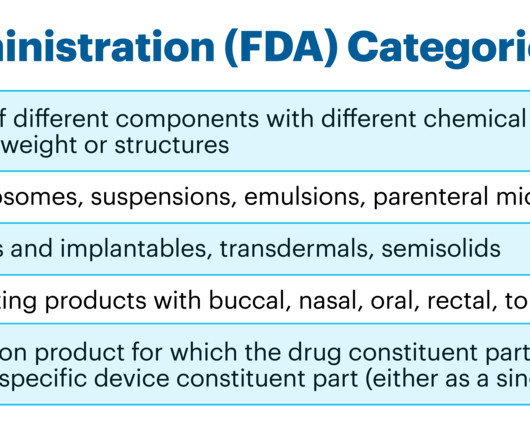
Labelling Requirements A ll dispensed medicine should be labelled according to the requirement stated by the law. NOTE: It is advisable for labels to be printed. Consequently, a patient-centred label strategy is introduced to promote appropriate medication use and adherence. Suggestions include Use larger font sizes (e.g.





































Let's personalize your content