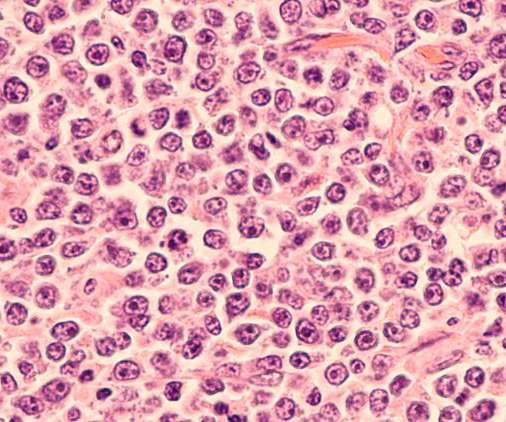
US opioid activists argue against Sackler family immunity in Purdue Pharma deal
The Guardian - Pharmaceutical Industry
DECEMBER 4, 2023
Under a court reorganization plan, Sackler family members who controlled the Oxycontin maker were granted immunity and did not have to declare personal bankruptcy in exchange for a $6bn settlement, paid out over 10 years, to a fund created to offset costs created by the opioid dependency crisis that has cost hundreds of thousands of lives.


































Let's personalize your content