What is Digital Communication: Comprehensive Explanation with Examples
Viseven
JUNE 4, 2025
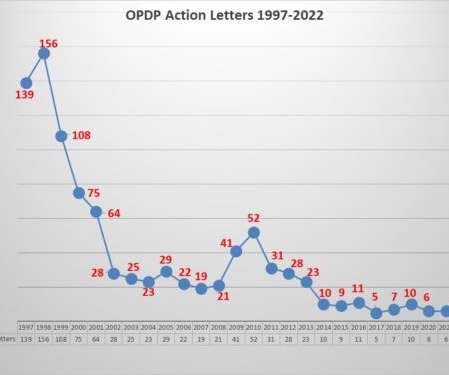
Have you ever stopped to wonder how your communication methods are shaping patient loyalty? A 2024 survey from Bandwidth revealed that nearly half of patients would consider switching healthcare providers if their communication needs weren’t met. What is Digital Communication?











































Let's personalize your content