STAT+: Pharma’s reputation with patient groups dips again, mostly due to pricing issues
STAT
APRIL 28, 2025
In 2021, for instance, 59% of companies scored highly, compared with 41% in 2018. The back-to-back declines come after 60% of patient groups believed the industry fared well thanks to the initial response of many companies to quickly develop salves for the Covid-19 pandemic. Continue to STAT+ to read the full story…




























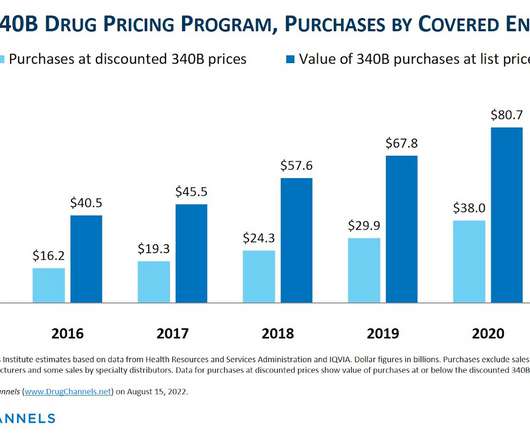
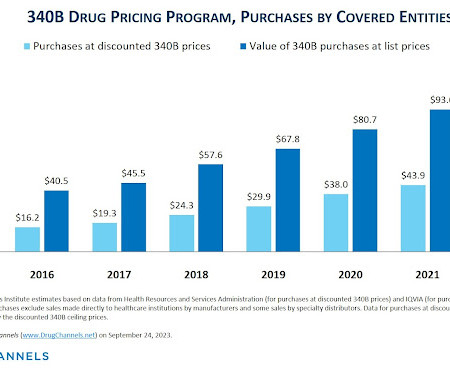

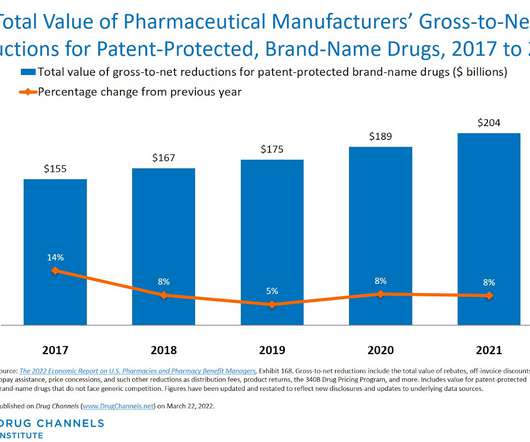

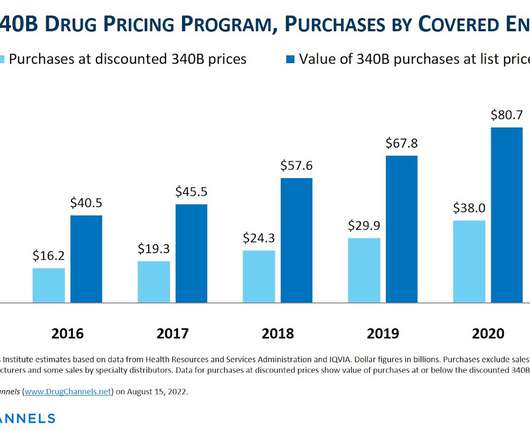













Let's personalize your content