BEAM-101 Receives RMAT for the Treatment of Sickle Cell Disease
Pharmacy Times
AUGUST 14, 2025
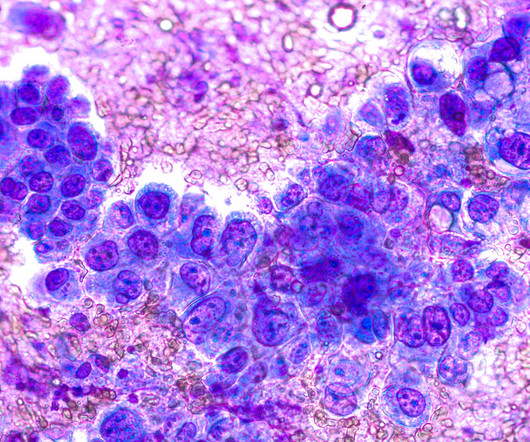
Completion Date (Estimated): February 1, 2027 BEAM-101 is an investigational, genetically modified cell therapy that consists of autologous CD34+ hematopoietic stem and progenitor cells that have been base-edited in the promoter regions of the HBG1/2 genes. Worldwide, about 8 million people have the disease.










Let's personalize your content