Govt provides updates on measures to encourage domestic manufacturing
Express Pharma
DECEMBER 9, 2024
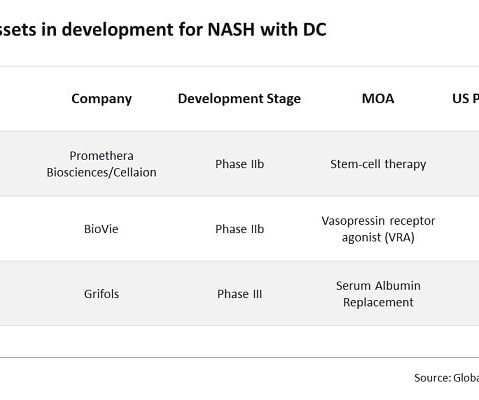
15,000 crores and a production tenure from FY 2022- 2023 to FY 2027-28, provides financial incentives to 55 selected applicants for manufacturing of identified products under three categories for six years. PLI Scheme for Pharmaceuticals, with a financial outlay of Rs. are manufactured. An incentive amount of Rs.3,215


















Let's personalize your content