EU’s Pharma Package falls short of Europe’s goal of global competitiveness
Pharmaceutical Technology
JULY 1, 2025
Further, they say the balance attempted between encouraging innovation and improving access instead could be contradictory for drug developers and investors, in a way that is emblematic of a fragmented EU. Industry reactions have been mixed.







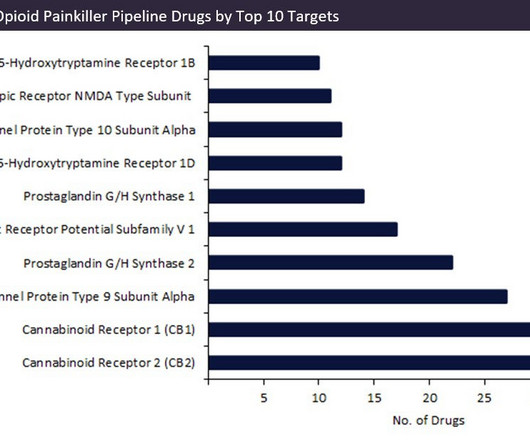









Let's personalize your content