Gerresheimer enhances pharma packaging registration with comprehensive product database
Express Pharma
JUNE 18, 2025
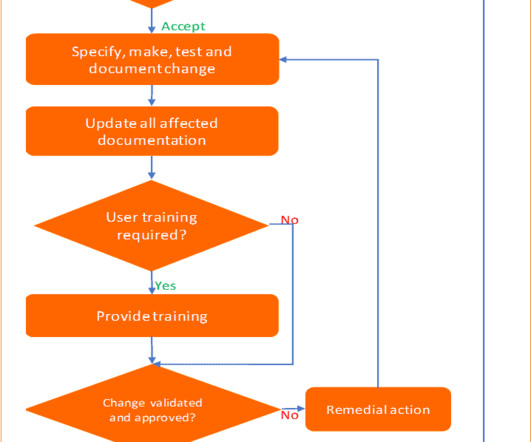
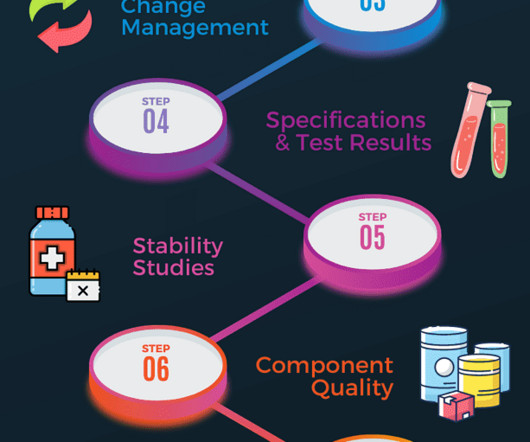
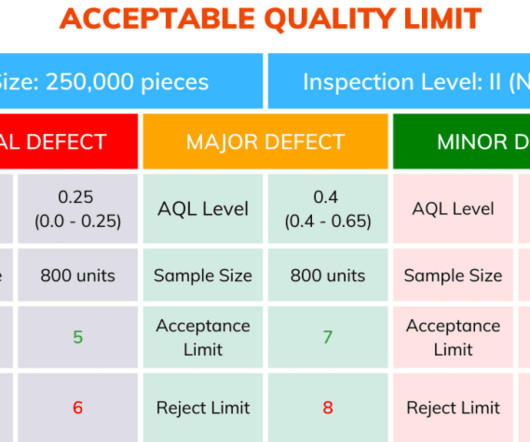
Gerresheimer, a systems and solutions provider for the pharmaceutical and biotech industry, has introduced a web-based product database designed to streamline the selection and registration of primary plastic packaging for medicinal products. It contains revision dates, test results, certifications, and regulatory documentation.











































Let's personalize your content