FOPE and PharmaState Academy hosts Session 11 of the PULSE series
Express Pharma
DECEMBER 23, 2024
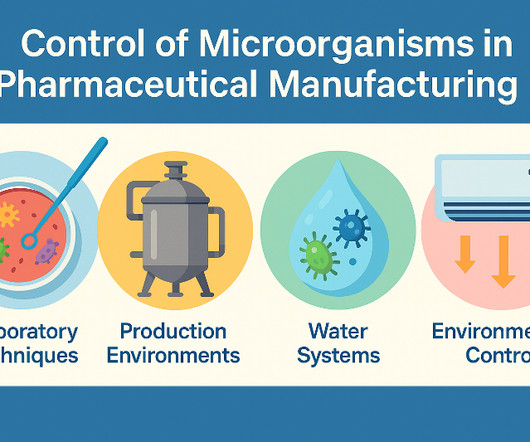
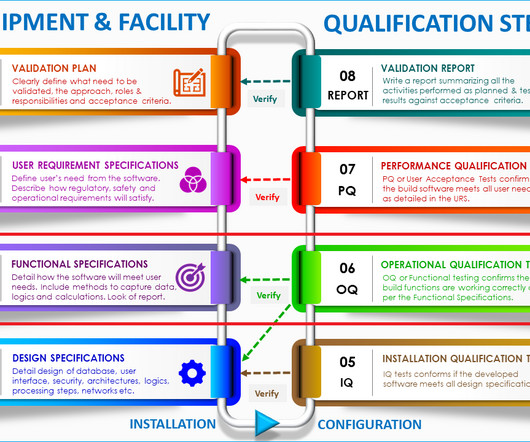
He presented on good practices in quality control, highlighting the importance of appropriate qualifications and experience, and adequate facilities for storage and sampling. He mentioned the necessity of having license number, and manufacturer address on certificates of analysis.
























Let's personalize your content