GE HealthCare’s PET imaging agent gains Alzheimer’s label expansion
Pharmaceutical Technology
JUNE 25, 2025
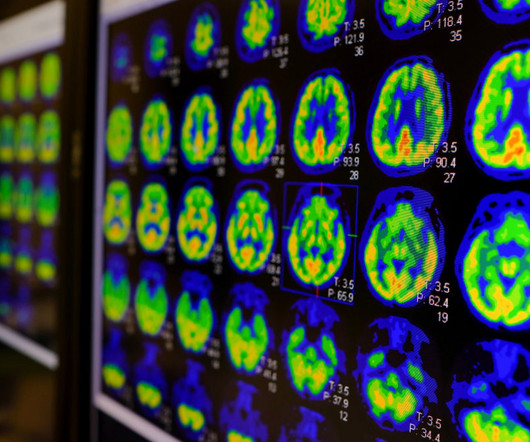
The updated label for GE HealthCare’s positron emission tomography (PET) imaging agent Vizamyl (flutemetamol F 18 injection) now includes quantification of amyloid in the brain, meaning patients taking an anti-amyloid therapy can be monitored for the drug’s effectiveness. Don’t let policy changes catch you off guard.

















Let's personalize your content