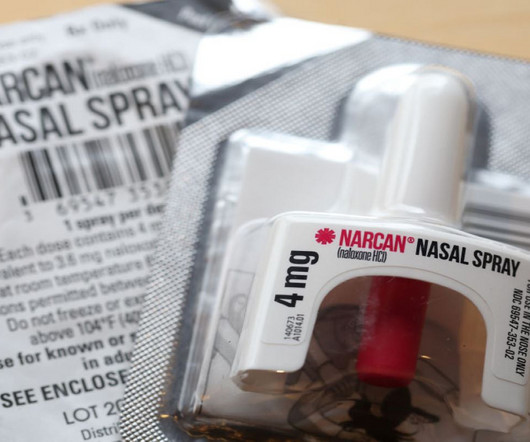
FDA tackles opioid crisis with OTC Narcan approval
Pharmaceutical Technology
MARCH 30, 2023
The overdose death rate in the US increased more than 250% from 1999 to 2019, as per a Canada-US joint white paper on substance use and harms. According to Emergent , the delay is due to manufacturing changes that will need to be made to support nonprescription packaging, and supply chain modifications.





















Let's personalize your content