FOPE and PharmaState Academy hosts Session 11 of the PULSE series
Express Pharma
DECEMBER 23, 2024
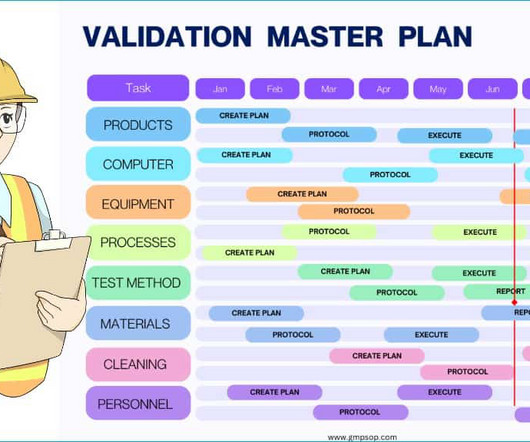
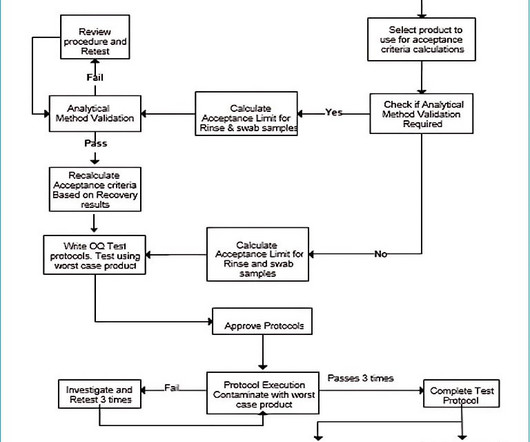
The panel also discussed the role of analytical method validation when outsourcing testing and the necessity of Excel sheet validation. The panellists patiently addressed participants’ questions on quality control practices in pharmaceutical manufacturing, particularly in the microbiological and chemical analysis sections.












Let's personalize your content