Cleaning validation protocol for pharmaceutical industry
GMPSOP
SEPTEMBER 27, 2023
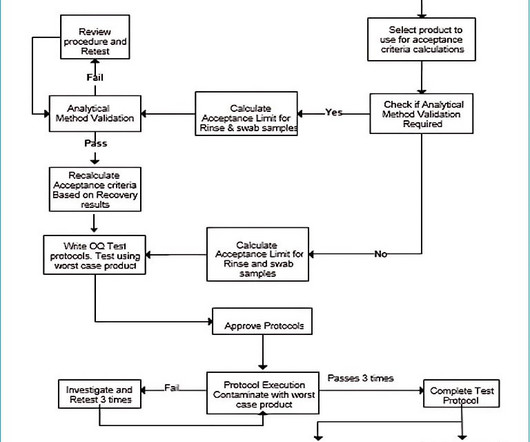
Cleaning validation verifies that the cleaning procedure can consistently and significantly reduce the amount of active ingredients, excipients, and cleaning agents to a concentration within the acceptance limit. Why is cleaning validation required? If it is not, analytical method validation is required.







Let's personalize your content