FOPE and PharmaState Academy hosts Session 11 of the PULSE series
Express Pharma
DECEMBER 23, 2024
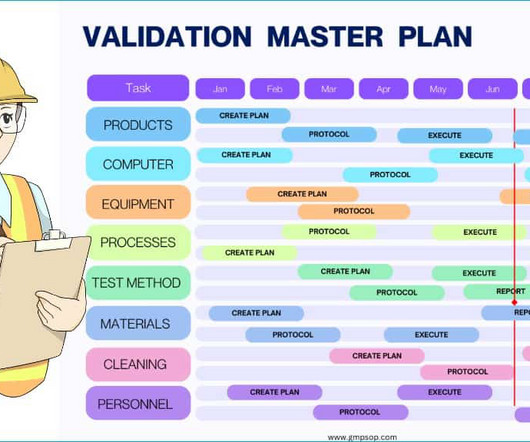
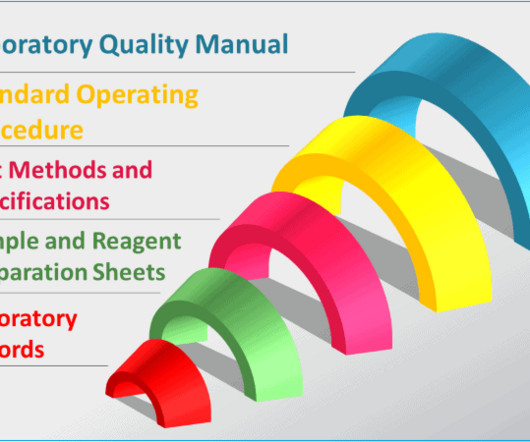
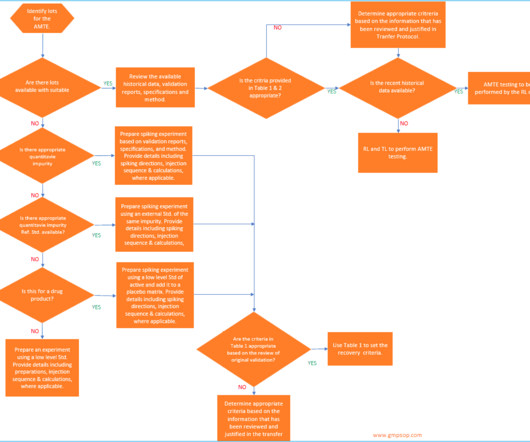
Dr Dikshit discussed the importance of validation in the quality control process, emphasising the need for user requirement specifications for standard equipment. He presented on good practices in quality control, highlighting the importance of appropriate qualifications and experience, and adequate facilities for storage and sampling.














Let's personalize your content