Smaller Pharmacies, Bigger Impact: Inside Manufacturers’ Specialty Networks in 2025
Drug Channels
APRIL 23, 2025
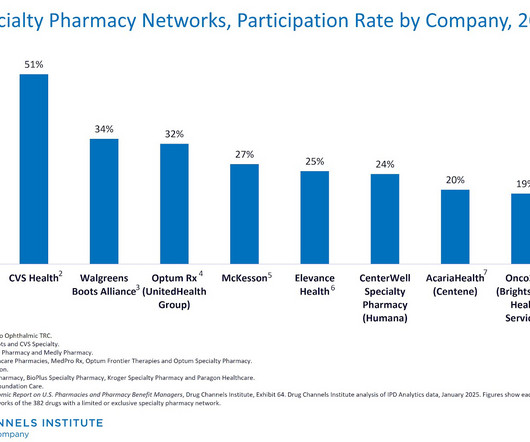
Last week, we examined the growing concentration of specialty drug dispensing revenues among the largest pharmacies. See The Top 15 Specialty Pharmacies of 2024: How PBMs, Health Systems, and Independents Are Shaping the Market. Today, we dive deeper. Drawing from DCIs new 2025 Economic Report on U.S.






















Let's personalize your content