What They Said – Looking Back the First 6-Months of FDA Communications
Eye on FDA
AUGUST 17, 2022
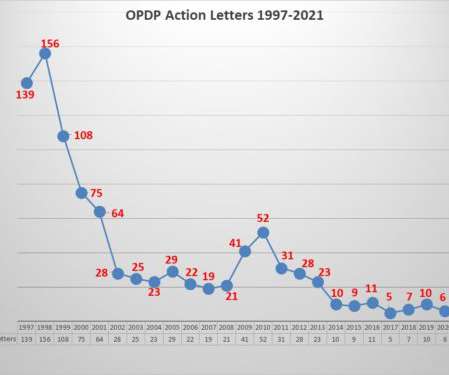
You can see early in the chart, FDA maintained a very low volume of communications, and at mid-year in 2017 had issued only 52 releases, rising to 166 for the year. The current downturn in volume could also be attributable in part to the fact that FDA has once again altered somewhat a past communications practice.

























Let's personalize your content