EMA publishes updated Q&A for ICH M10
European Pharmaceutical Review
JANUARY 26, 2023
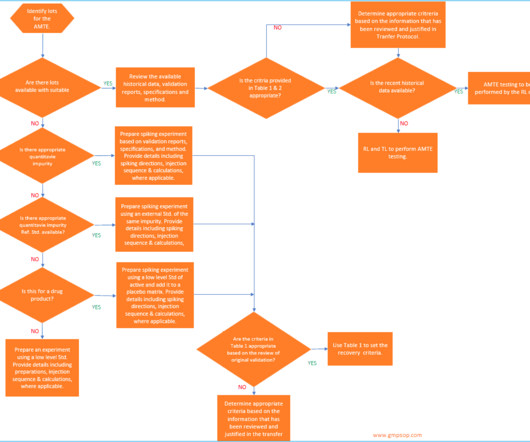
The European Medicines Agency (EMA) has published an updated Q&A document regarding ICH M10 ‘Bioanalytical Method Validation and Study Sample Analysis’. The ICH M10 guideline provides recommendations on the validation of bioanalytical assays for chemical and biological drugs and their metabolites in biological matrices.












Let's personalize your content