How to conduct product quality review in pharmaceutical
GMPSOP
NOVEMBER 19, 2023
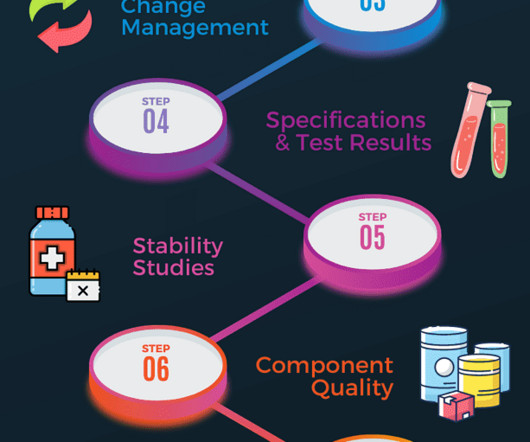
Component quality A review of all starting materials and primary packaging in contact with the product should be completed. This will include supplier performance assessment and identifying any critical deviations associated with active or excipient ingredients, primary packaging and closure material. Subscribe f.









Let's personalize your content