Multi-sector pharmacist training no longer mandatory for 2026/27
The Pharmacist
DECEMBER 17, 2024
Pharmacy employers will not have to offer multi-sector training placements in 2026/27, NHS England (NHSE) has announced.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
 2026 Related Topics
2026 Related Topics 
The Pharmacist
DECEMBER 17, 2024
Pharmacy employers will not have to offer multi-sector training placements in 2026/27, NHS England (NHSE) has announced.

The Pharmacist
NOVEMBER 21, 2024
The government has published draft legislation outlining a ‘permanent’ cut in business rates for retail, hospitality and leisure (RHL) properties from 2026, including pharmacies. The tax cut will be funded by a tax rise for the very largest business properties, such as online sales warehouses.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

STAT
OCTOBER 29, 2024
billion in estimated out-of-pocket savings for people with Medicare prescription drug coverage when the negotiated prices are set to go into effect in 2026.

The Pharmacist
JULY 3, 2025
Community pharmacies will be able to provide the HPV vaccine from 2026 to those who missed out at school, according to the government’s 10-year health plan, published today.

STAT
MAY 1, 2025
CVS Health will not sell its Aetna health plans in the Affordable Care Act’s individual marketplaces in 2026, marking the second time in the past decade that Aetna has given up on ACA coverage. CVS expects to lose up to $400 million this year in its ACA plans.

Med Ed 101
MAY 28, 2025
What a waste […] The post Uniform MPJE From NABP – Coming In 2026 appeared first on Med Ed 101. I have licenses in 3 states and that is plenty for me to keep track of. Some pharmacists are having to obtain double-digit licenses to perform their role as a pharmacist.

Express Pharma
APRIL 8, 2025
Steris Healthcare, a pharmaceutical company headquartered in Mumbai with operations in Navi Mumbai, has unveiled a 50 crore growth strategy aimed at expanding its manufacturing capacity, entering new regional markets, and launching an Initial Public Offering (IPO) in FY 202627.

STAT
JANUARY 29, 2025
Curie.Bio’s new fund will help the organization double its investing over the next two years: The aim is to invest in between 15 and 20 biotechs this year and up to 25 companies in 2026, the founders told STAT exclusively. Continue to STAT+ to read the full story…

National Association of Boards of Pharmacy
APRIL 16, 2025
Uniform MPJE Launches Spring 2026 Although the content outline will be released soon, the uniform version of the MPJE is expected to launch in June 2026. The content outline for this new exam will be released in May 2025. The development process will remain responsive to the evolving needs of the pharmacy community.

The Pharmacist
NOVEMBER 26, 2024
Pharmacists have been urged to continue to promote the importance of folic acid supplementations following news that it will be added to non-wholemeal flour from the end of 2026.

Putting Patients First Blog
APRIL 10, 2025
NHC Statement on Fiscal Year 2026 Federal Health Care Funding NHC Statement on Fiscal Year 2026 Federal Health Care Funding (PDF) Prepared written testimony for Kimberly Beer Senior Vice President, Policy and External Affairs, National Health Council U.S.
Express Pharma
DECEMBER 2, 2024
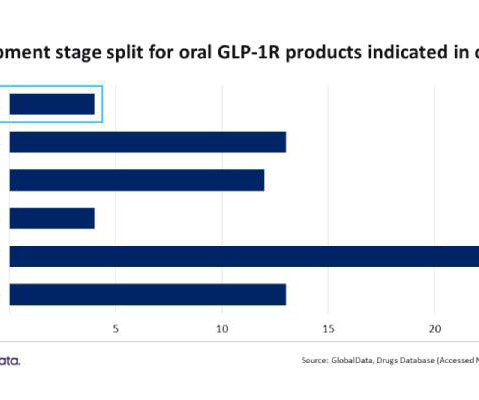
With a projected launch in 2026, it represents Eli Lilly’s bid to expand the company’s dominance in the GLP-1R category.” Morley adds, “Eli Lilly’s lone Phase III candidate, orforglipron calcium, has two firsts, becoming the initial oral GLP-1R approved for obesity and achieving the first GLP-1R approval for a small molecule.
Fuld & Company Blog
OCTOBER 3, 2024
Set to be operational by 2026, the project will produce 2,800 tons of green hydrogen annually. This 50:50 joint venture will involve the construction of a 25 MW electrolyzer powered by renewable electricity, which will support the decarbonization of BP’s refining operations.

Express Pharma
DECEMBER 26, 2024
The site and product dossier approvals are expected to be received by the end of 2026. As part of this initiative, Akums Group will initiate the European approval process for its oral liquid manufacturing facility, leveraging this approval to produce the contracted products.

European Pharmaceutical Review
JULY 10, 2025
The European Pharmacopoeia’s decision to abolish rabbit pyrogen testing by 2026 exemplifies this trend” The pharmaceutical industry’s testing landscape continues to evolve, particularly where animal-derived methods face increasing scrutiny.

European Pharmaceutical Review
FEBRUARY 7, 2025
Data from the ongoing Phase III FRONTIER programme will be announced in 2025 and 2026, shared Novo Nordisk. This was compared to individuals not given prophylaxis treatment, according to Novo Nordisk. Regulatory submission for Mim8 is anticipated in 2025.

European Pharmaceutical Review
MARCH 19, 2025
The COMP006 26-week data is anticipated in the second half of 2026, according to Compass Pathways. COMP360 was found to be generally well tolerated by participants in the COMP004 study.

Beckers Hospital Review
JULY 18, 2025
Under the newly released 2026 outpatient rule, CMS said it plans to accelerate reimbursement cuts to 340B hospitals for non-drug items and services, according to a July 16 news release shared with Becker’s. The group said using cost survey data to set future payment rates could result in hospitals losing the benefit of 340B pricing.

National Association of Boards of Pharmacy
JUNE 26, 2025
The Pre-uniform MPJE is expected to be available in early 2026. In 2026, the Pre-uniform MPJE will only provide a raw/percentage correct result and will not provide a scaled score result. Yes, NABP plans to follow the established exam development process to create a practice exam for the uniform version of the MPJE.

The Pharmacist
JULY 10, 2025
The recall affects batch number 4V64 (expiry date: 30 September 2026), distributed in 5ml packs. Pharmacists have been advised to stop supplying one batch of Zaditen 0.25 mg/ml eye drops solution following a precautionary recall issued by the Medicines and Healthcare products Regulatory Agency (MHRA).

ID Stewardship
APRIL 16, 2025
Candidate 2026 Article posted 16 April 2025 No one really warns you about just how much content you will encounterin pharmacy school – until you are in it. In this article a current third year pharmacy student discusses pharmacy student study strategies to maximize learning in 2025 and beyond. Authored By:Fabian Quiroga, Pharm.D.

Beckers Hospital Review
JULY 14, 2025
CMS has unveiled a proposed rule for the 2026 Medicare Physician Fee Schedule, including a new ambulatory specialty care model aimed at improving chronic disease management and reducing costs. CMS has proposed two separate conversion factors for 2026 to reflect statutory changes and adjustments for proposed work relative value units.

PharmaShots
JUNE 11, 2025
Shots: The US FDA has approved the IND application of NVC-001 to treat LMNA-related dilated cardiomyopathy (LMNA DCM), enabling a P-I/II trial to start in early 2026 Preclinical studies showed that NVC-001 significantly improved survival and cardiac function.

Pharmaceutical Technology
JUNE 19, 2025
BioOrbit aims to kick off pre-clinical trials to study protein crystals for monoclonal antibodies (mAbs) produced in a pharmaceutical factory in space in 2026. Then, we will need to run pre-clinical trials of these crystals, which will be in 2026 or 2027. Image credit: Rini. com/ Shutterstock.
Pharmaceutical Business Review
APRIL 8, 2025
Initial safety data from these trials are expected to be available in the first half of 2026. Based on the FDA clearance, the company anticipates the commencement of Phase I clinical trials for ALX2004 in mid-2025.

Pharmacy Times
JUNE 27, 2025
Mary Dzhuryan is a class of 2026 PharmD candidate at the USC Alfred E. Mehrnaz Razavi Vakhshoori is a class of 2026 PharmD candidate at the USC Alfred E. The recent application was granted both priority review and orphan drug designation. Mann School of Pharmacy and Pharmaceutical Sciences in Los Angeles.

Express Pharma
JANUARY 10, 2025
The new joint offering from Kindeva and Emervax is anticipated to move into clinical trials in 2026 and is projected to help fight infectious diseases, including, Yellow Fever, Ebola, Monkeypox, Tuberculosis, along with selected cancers and autoimmune diseases, as per a release.

Pharmaceutical Business Review
JUNE 11, 2025
We look forward to initiating a Phase 1 healthy volunteer study in the coming weeks, with clinical results expected in H1 2026, including data on safety, pharmacokinetics, NEK7 protein degradation, and downstream pharmacodynamic markers. “As The company expects to submit an IND for this programme in 2026.
Express Pharma
DECEMBER 9, 2024
3,420 crore and production tenure from FY 2022-2023 to FY 2026-27, provides an incentive to selected companies at the rate of 5 per cent on incremental sales of medical devices manufactured in India and covered under the four target segments of the scheme, for five years. are manufactured.

European Pharmaceutical Review
MAY 20, 2025
Once fully implemented in April 2026, the] reforms will address the research sectors need for a more risk-proportionate regulatory framework for clinical trials and will help get cutting-edge new treatments to the NHS as quickly as possible, explained Lawrence Tallon , MHRA Chief Executive.

Pharmacy Times
JUNE 4, 2025
USP plans to continue expanding its advanced technology footprint into an additional 8,000 square foot laboratory in 2026. The Advanced Technologies Lab is the latest of USP’s investments in advanced manufacturing technologies. Learn more about USP’s supply chain solutions.
Pharmacy Times
MAY 27, 2025
Following a meeting of the Vaccines and Related Biological Products Advisory Committee, the FDA announced their recommendation of COVID-19 vaccines targeting LP.8.1, a strain of the JN.1 1 variant that has become dominant in the US.

Pharmacy Times
JULY 10, 2025
Related Videos Related Content Advertisement June 11th 2025 Study Finds Depression May Increase Risk of Developing Dementia Gillian McGovern, Associate Editor July 10th 2025 Pharmacy Focus: Importance of Mental Health for Patients and Pharmacists Alike Gustavo Alva, MD Luke Halpern, Assistant Editor June 8th 2025 Ambulatory Pharmacy in Action: House (..)

Pharmacy Times
JULY 4, 2025
Related Videos Related Content Advertisement July 4th 2025 Shingles and RSV Vaccines Show Promise Against Dementia Kennedy Ferruggia, Assistant Editor May 28th 2025 Pharmacy Focus: Navigating Seasonal Allergies Luke Halpern, Assistant Editor Derek Webb, PharmD July 2nd 2025 FDA Issues Complete Response Letter to Manufacturer of OLC, Treatment for CKD-Related (..)

The Pharmacist
JULY 18, 2025
However, plans for an additional rise in 2026 have been put on hold to allow for further reflection on concerns raised by registrants. The General Pharmaceutical Council (GPhC) will push forward with a 6% increase in its annual registration fees this year despite strong opposition from the profession.

Express Pharma
APRIL 10, 2025
It is a critical milestone for our company as Brazil is one of the biggest markets opening for generic Semaglutide in 2026. This approval now enables OneSource to supply pharmaceutical products specially DDCs, including GLP-1s manufactured at this site to the Brazilian market upon our customers getting their product approvals.

The Pharmacist
MAY 30, 2025
Applications for the training courses which are available until March 2026 are open across several universities throughout England, with different intake start dates available. NHS England is funding 3,300 places on independent prescribing courses for pharmacists for 2025/26, it has announced.
Pharmaceutical Commerce
JUNE 9, 2025
Image Credit: Adobe Stock Images/Artur.com Key Takeaways Sanofi is shipping Beyfortus early to ensure strong supply for the 2025–2026 RSV season and avoid past shortages. Press Release: Sanofi accelerates global shipping of Beyfortus to prepare healthcare providers months ahead of 2025-2026 RSV season. 3 References 1. June 9, 2025.

Putting Patients First Blog
JUNE 26, 2025
NHC Comments on Medicare Drug Price Negotiation Program: Draft Guidance, Implementation of Sections 1191–1198 of the Social Security Act for Initial Price Applicability Year 2027 and Manufacturer Effectuation of the Maximum Fair Price (MFP) in 2026 and 2027. July 2, 2024. link] 2027_07.02.24.pdf. 51 National Health Council. April 14, 2023.

Express Pharma
DECEMBER 18, 2024
The number of post-menopausal women in the country is projected to rise from 96 million in 2011 to an estimated 401 million by 2026. The launch of Miror Bliss reflects the growing demand for targeted wellness solutions in India.

Express Pharma
FEBRUARY 9, 2025
The study is expected to complete enrollment between Q4 2025 and Q1 2026. In the U.S., the Phase I/II GLIOSTELLA trial is enrolling patients with glioblastoma at first or later progression, with 36 out of 90 patients recruited so far.

BioPharma Dive
JULY 9, 2025
The company is “preparing for clinical development in 2026” across multiple indications, Goutopoulos said. Some of those, including Novartis and Ratio Therapeutics , are also targeting FAP. Actithera has not disclosed which cancers it’s targeting with its therapies.

PharmaVoice
JULY 8, 2025
Whether the results — and Immuneering’s approach — will hold up in a pivotal trial planned for 2026 remains to be seen. The results marked a major step for a disease with a 67% six-month survival rate using standard chemotherapies. And if you can do that, cancer becomes a chronic, manageable disease, like diabetes, cardiac disease or HIV.

European Pharmaceutical Review
JUNE 6, 2025
Dr Pakola shared that the trial is anticipated to support a Biologics License Application (BLA) submission for the gene therapy under accelerated approval in mid-2026. Top-line data is expected in H1 of 2026. The post Duchenne gene therapy interim trial outcomes “striking” appeared first on European Pharmaceutical Review.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content