Pharma contract manufacturing market to grow to $130bn by 2026
European Pharmaceutical Review
AUGUST 19, 2022
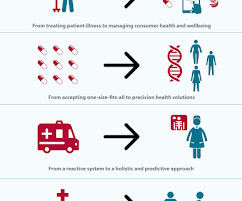
billion by 2026. percent compound annual growth rate (CAGR) of the market include the increasing commercialisation of generics and emerging technologies and small biotechs with limited or no production capabilities driving development in the biopharmaceutical sector. percent between 2021 and 2026 (the forecast period).





















Let's personalize your content