NHC Submits Comments on CMS Draft Guidance for IPAY 2028
Putting Patients First Blog
JUNE 26, 2025
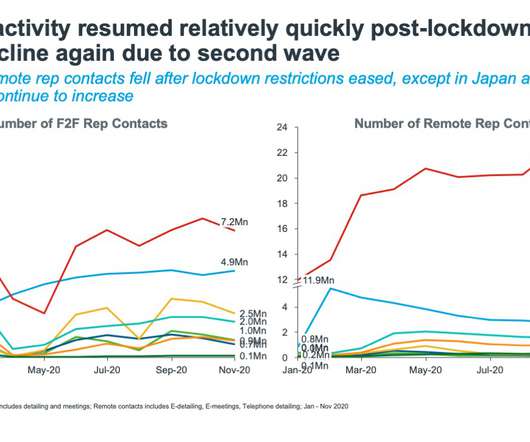
Inclusion of Part B Drugs: Scope, Challenges, and Opportunities The addition of provider-administered therapies covered under Medicare Part B to the IPAY 2028 cycle introduces a fundamentally different reimbursement and care delivery context than that of pharmacy-dispensed Part D drugs.












Let's personalize your content