FDA revises biosimilar guidelines for clearer drug labelling
Pharmaceutical Technology
SEPTEMBER 18, 2023
The FDA released a draft guidance giving advice on the correct labelling of biosimilar and interchangeable biosimilar products.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
SEPTEMBER 18, 2023
The FDA released a draft guidance giving advice on the correct labelling of biosimilar and interchangeable biosimilar products.
STAT
NOVEMBER 28, 2022
The provision is known as skinny labeling , which refers to a move by a company that seeks regulatory approval to market a generic or biosimilar medicine for a specific use, but not for other patented uses for which the brand-name drug is prescribed. billion from 2015 to 2020 — or nearly 5% of the $30.2
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
pharmaphorum
AUGUST 2, 2023
Samsung seeks 'interchangeable' label for Humira biosimilar Phil.Taylor Wed, 02/08/2023 - 10:37 Bookmark this

pharmaphorum
AUGUST 25, 2023
CVS Health launches private label biosimilars unit Phil.Taylor Fri, 25/08/2023 - 08:07 Bookmark this

Pharmaceutical Commerce
SEPTEMBER 19, 2023
The draft guidance document discusses the development of labeling for proposed biosimilars and interchangeable biosimilars for submission under section 351(k) of the Public Health Service Act.

PharmaTech
SEPTEMBER 18, 2023
The draft guidance document discusses the development of labeling for proposed biosimilars and interchangeable biosimilars for submission under section 351(k) of the Public Health Service Act.

Big Molecule Watch
SEPTEMBER 25, 2023
Last week, FDA released a draft guidance, “ Labeling for Biosimilar and Interchangeable Biosimilar Products ” (“2023 Draft Guidance”) that—when finalized—will revise and replace its July 2018 final guidance, “ Labeling for Biosimilar Products.”

Safe Biologics
DECEMBER 19, 2023
The guidance removed the interchangeability statement from the product label/package insert of interchangeable biosimilars. state law, only interchangeable biosimilars may be substituted by a pharmacist without contacting the prescriber. The agency has approved 44 biosimilar products, including seven interchangeable biosimilars.
European Pharmaceutical Review
MARCH 6, 2024
The US Food and Drug Administration (FDA) has approved the first and only FDA-approved denosumab biosimilars, to treat all indications of the reference medicines. The FDA noted that its decision for the biosimilars is based on clinical study data, which showed no clinically meaningful differences from the reference medicines.

FDA Law Blog: Biosimilars
APRIL 25, 2024
This Revised Draft Guidance provides considerations for manufacturers, packers or distributors (dubbed “firms”) of prescription biological reference products, biosimilar products, and interchangeable biosimilar products presenting data and information about such products in promotional materials in a truthful and non-misleading way.
Drug Channels
OCTOBER 3, 2023
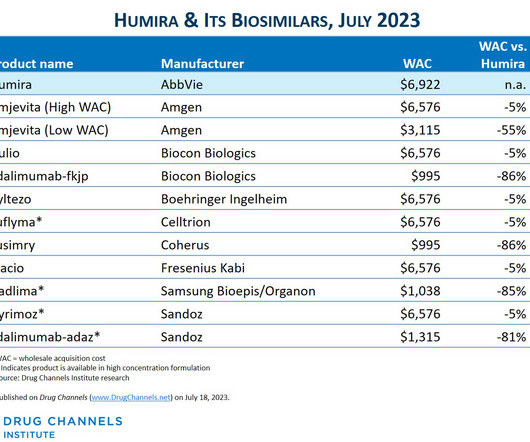
Since I published the article below in July 2023 , there have been three notable market develpoments: IQVIA has reported that as as of mid-2023, there was almost no adoption of Amgen's Amjevita, the first Humira biosimilar. Boehringer-Ingelheim launched an unbranded, low WAC version of its interchangeable biosimilar.

Drug Channels
DECEMBER 11, 2023
One update: CVS Caremark's 2024 formulary for patient-administered autoimmune products prefers: Humira Hyrimoz (the high WAC Sandoz biosimilar) adalimumab-adaz (the unbranded, low WAC Sandoz biosimilar) Perhaps coincidentally, Sandoz products are marketed by Cordavis, the new business described in today's rerun.

pharmaphorum
AUGUST 24, 2022
Amgen has reported positive phase 3 results with its biosimilar version of AstraZeneca/Alexion’s blockbuster rare disease drug Soliris, setting up a regulatory filing with the FDA. The safety and immunogenicity profile of the biosimilar was also comparable to Alexion’s drug, said Amgen.

Pharmaceutical Technology
AUGUST 25, 2023
The Sandoz biosimilar, Tyruko, was approved by the US FDA for all labelled indications of Tysabri based on Phase I and III data.

Drug Channels
SEPTEMBER 6, 2023
CVS Health has finally revealed its strategy for biosimilars of AbbVie’s Humira. Rather than announce multiple biosimilars for its pharmacy benefit manager (PBM) formulary, the company will instead launch Cordavis, a new subsidiary that will market a private label, low-list-price version of Sandoz’ Hyrimoz.

Big Molecule Watch
AUGUST 24, 2023
In continuation of Goodwin’s previous webinar series concerning biosimilars, Big Molecule Watch is launching the 2023-2024 webinar series , which will dive deep into some of the key topics pertaining to this burgeoning industry and corresponding area of law. The post Biosimilars Webinar Series appeared first on Big Molecule Watch.

FDA Law Blog: Biosimilars
MAY 11, 2023
GSK skinny label case , the U.S. Specifically, the Government explained, “[t]he section viii pathway cannot function properly if FDA and generic manufacturers cannot rely on an NDA holder’s representations to the agency regarding which portions of the brand-name drug’s labeling teach patented methods of use.”

FDA Law Blog: Biosimilars
MARCH 5, 2024
Many individual states have pursued some type of legislation to restrict the use of traditional meat terminology for the labeling of APPs. Many states have proposed but failed to enact legislation regulating the labeling of APPs, in some cases due to concerns of potential legal challenges based on federal preemption claims.

Safe Biologics
DECEMBER 3, 2023
ASBM and GaBI Webinar Examines Policy Challenges to Interchangeable Biosimilars On November 30, ASBM and the Generics and Biosimilars Initiative (GaBI) hosted I nterchangeable Designation for Biosimilars- Ensuring Continuity of Patient Care: Upholding Interchangeability Status for Biosimilars.

Big Molecule Watch
SEPTEMBER 7, 2023
Developing and successfully commercializing a biosimilar is a complex and expensive process. Topics that will be covered include the 271(e) Safe Harbor, peremptory challenges, labelling and timing considerations, and competitive intelligence. Click here to register for the webinar. Click here to register for the webinar.

FDA Law Blog: Biosimilars
APRIL 2, 2024
In the first category, FDA asks Congress to amend the FDCA to require drug manufacturers to disclose full information about the name and quantity of inactive ingredients in product labeling and permit FDA to disclose to generic sponsors the names and amounts of such inactive ingredients.
pharmaphorum
SEPTEMBER 20, 2021
Samsung Bioepis and Biogen have claimed the first FDA approval for a biosimilar version of Roche and Novartis’ Lucentis (ranibizumab) for leading causes of blindness, raising the prospect of a cheaper treatment option for US patients. The post FDA OKs first biosimilar of Roche’s blockbuster AMD drug Lucentis appeared first on.

FDA Law Blog: Biosimilars
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts. A recent state law failure-to-warn case in the SDNY makes that very point.

pharmaphorum
NOVEMBER 19, 2020
Novartis/Genentech’s eye drug Lucentis could be the next big blockbuster to face competition from cheaper biosimilars after its US patent expired this year – and Samsung Bioepis and Biogen are closing in after the FDA accepted a filing for their cut-price rival.

Safe Biologics
NOVEMBER 12, 2023
6 “The Biosimilar Red Tape Elimination Act”, which would prevent the HHS Secretary from requiring switching studies in order for a biosimilar to be deemed “interchangeable” Under U.S. state law, only biosimilars which are interchangeable may be substituted by a pharmacist without contacting the prescriber.

Big Molecule Watch
NOVEMBER 21, 2023
District Court for the District of New Jersey, alleging infringement of 15 patents under the BPCIA based on DRL’s submission of an aBLA for DRL_RI, a proposed biosimilar of RITUXAN (rituximab) and DRL’s provision of Notice of Commercial Marketing with respect to the same. Stay tuned to Big Molecule Watch for more updates on this litigation.

Safe Biologics
DECEMBER 6, 2023
prescribers have high confidence in the safety and efficacy of biosimilars, a majority (58%) oppose third-party switching of a patient’s biologic medicine for non-medical (e.g. state law, only biosimilars which are interchangeable may be substituted by a pharmacist without contacting the prescriber. and worldwide.”

FDA Law Blog: Biosimilars
AUGUST 17, 2023
Vanda requested that FDA revoke the approval of Apotex’s and Teva’s generic versions of Hetlioz on the grounds that the generic tasimelteon products did not meet the statutory “same labeling” requirement for generic drugs found in 21 U.S.C. § Vanda’s Hetlioz was, in fact, the first FDA-approved drug product to include braille labeling.

FDA Law Blog: Biosimilars
MAY 23, 2023
Food and Drug Administration (FDA) released a draft update to its Compliance Policy Guide (CPG) for FDA staff on the Agency’s enforcement of major food allergen labeling and cross-contact. The draft CPG directs FDA field staff to examine possible food product adulteration due to labeling related to allergen cross-contact.

Big Molecule Watch
JANUARY 25, 2024
As we kick off 2024, we reflect on regulatory developments in the biologics and biosimilars space in 2023. The approval of Amgen’s WEZLANA (ustekinumab-auub) as biosimilar to Janssen’s STELARA (ustekinumab) is noteworthy given that it received designation as interchangeable. Below are some of the top regulatory developments from 2023.

FDA Law Blog: Biosimilars
MAY 23, 2023
We previously noted that “ the skinny label may be dead ” and, while we still can’t be sure if it’s truly gone (but not forgotten), we now know that the Supreme Court won’t hear this case at this time. So, while the skinny label may not actually be dead, there will certainly be a reluctance to use it.

pharmaphorum
JULY 26, 2021
Fresh from its takeover of Alexion, AstraZeneca has picked up a recommendation in the EU for an expansion of the label of Ultomiris, one of the main assets behind the $39 billion merger. The post Boost for AZ as Ultomiris gets CHMP nod for expanded label appeared first on.

PharmaShots
FEBRUARY 3, 2023
In preclinical studies, HLX15 is highly similar to daratumumab Ref: Henlius | Image: Henlius Related News:- Henlius' HLX15 (biosimilar- daratumumab) Receives IND Approval for Multiple Myeloma in China The 1EP is the area under the serum drug concentration-time curve from time 0 to infinity & 2EPs incl.

Big Molecule Watch
MARCH 27, 2023
Reddy’s”) to license Dr. Reddy’s proposed biosimilar abatacept for the development and commercialization of COYA 302 for the treatment of neurodegenerative conditions. On March 20, 2023, Coya Therapeutics, Inc. (“ Coya ”) announced a worldwide agreement with Dr. Reddy’s Laboratories Ltd. (“Dr.

FDA Law Blog: Biosimilars
OCTOBER 24, 2023
By Dara Katcher Levy — Yesterday, FDA published a new Draft Guidance, “ Communications from Firms to Health Care Providers Regarding Scientific Information on Unapproved Uses of Approved/Cleared Medical Products Questions and Answers ” (SIUU Guidance or Draft Guidance).

Safe Biologics
MARCH 30, 2023
Significantly for the INN group, a 2020 WHO report identified inconsistent nomenclature as a remaining challenge as it is clear that naming and labelling are both very important for pharmacovigilance and prescribing. Read the WHO’s summary of the 75th INN Consultation here.

FDA Law Blog: Biosimilars
APRIL 21, 2024
Accordingly, had Taiho marketed the product with labeling containing those errors, that labeling would have been false. Thus, regardless of the technicality of an initial notification letter stating approval on September 30, 2022, Taiho was prohibited under the FDCA from marketing LYTGOBI with false labeling, 21 U.S.C.

European Pharmaceutical Review
DECEMBER 20, 2023
Changes to regulatory data protection periods are of particular interest to biopharma” Changes to regulatory data protection (RDP) periods are of particular interest to biopharma (originators and generic / biosimilar manufacturers). analysis (Source: L.E.K) By contrast, orphan drugs could become less profitable, more risky investments.

PharmaShots
JUNE 13, 2023
mNSCLC, with No EGFR and ALK Genomic Tumor Published: 20 Aug,2018 | Tags: Merck, FDA, Expanded, Label, Approval, Mnsclc Organogenesis Reverse Merges with Avista Healthcare Public Acquisition Corp.

PharmaShots
JUNE 28, 2023
Regeneron is jointly developing aflibercept along with Bayer Ref: Regeneron | Image: Regeneron Related News:- Alvotech Entered into an Exclusive Agreement with Polifarma to Commercialize AVT06, a Proposed Biosimilar to Eylea (aflibercept) in Turkey PharmaShots! Your go-to media platform for customized news ranging for multiple indications.

Big Molecule Watch
JUNE 29, 2023
As we previously reported , Biogen sued Sandoz and Polpharma (“Defendants”) in a BPCIA litigation related to Defendants’ natalizumab biosimilar. The Court also noted that Biogen’s own expert cited sources showing that, in the 12-18 months after biosimilar launch, the price of the biosimilar product remained stable.

PharmaShots
JUNE 13, 2023
The safety & tolerability profiles were consistent throughout the primary treatment period & open-label extension Soliris (C5 complement inhibitor) was approved in the US, EU, Japan & China for PNH, aHUS & adults with gMG.
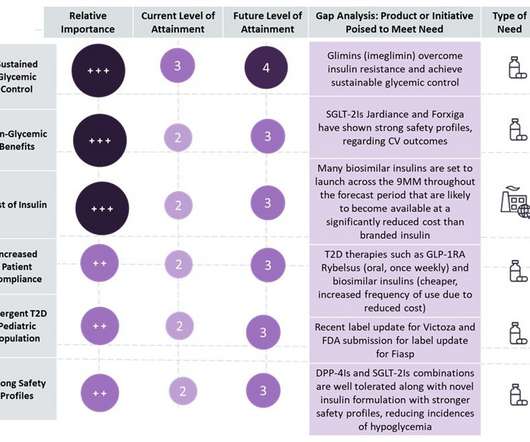
Pharmaceutical Technology
JANUARY 10, 2023
Type 2 diabetes (T2D) is a crowded and competitive landscape with multiple “me-too,” generic and biosimilar drugs entering the market, with market growth primarily driven by an increasing prevalent population across nine major markets (9MM: US, France, Germany, Italy, Spain, UK, Japan, China, and India).

FDA Law Blog: Biosimilars
JUNE 11, 2023
However, over time, data on pediatric labeling changes pursuant to BPCA and/or PREA have been collected. Between 2002 and 2019, there were 768 products with pediatric labeling changes under BPCA and/or PREA. Still, however, it appears FDA has had enough of these 16%.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content