Adverse Drug Reactions
RX Note
JULY 30, 2023
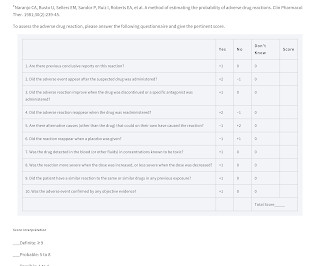
Adverse Drug Reactions The World Health Organization (WHO) defines an adverse drug reaction (ADR) as 'a drug-related event that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function'.









Let's personalize your content