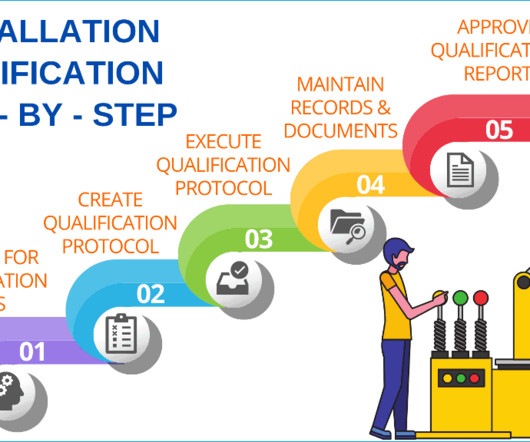
A step-by-step guide to successful installation qualification (IQ)
GMPSOP
JULY 23, 2023
Installation qualification will provide you with documented evidence that the equipment or system has been designed, developed, supplied, and installed in accordance with design drawings, the supplier’s recommendations, and in-house user requirements. Additional documents included each month. Checkout sample preview s.








Let's personalize your content