Corrective and Preventive Action (CAPA) Procedure for GMP
GMPSOP
MARCH 17, 2023
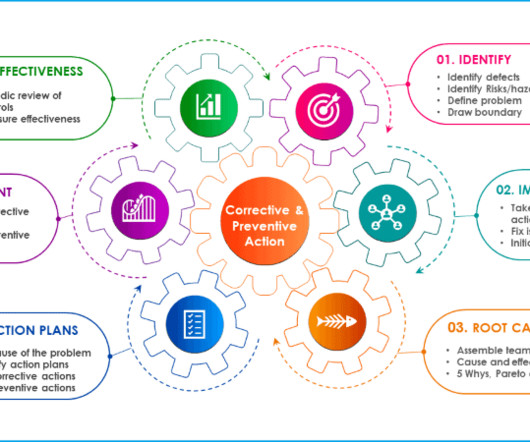
Immediately communicate the incident to stakeholders and initiate an internal laboratory investigation. Additional documents included each month. record who had found the incident first time) Corrective and preventive action process Embed Code 240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms.







Let's personalize your content