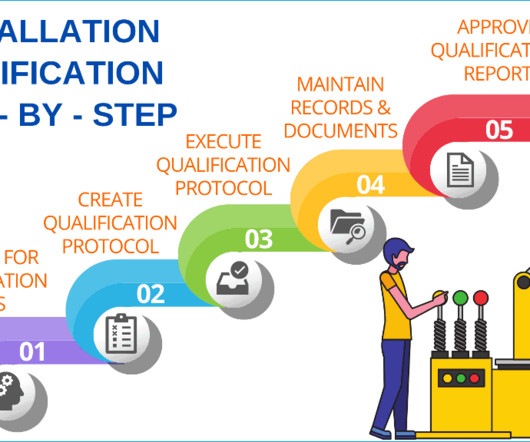
Concept of validation in pharmaceutical industry
GMPSOP
OCTOBER 26, 2022
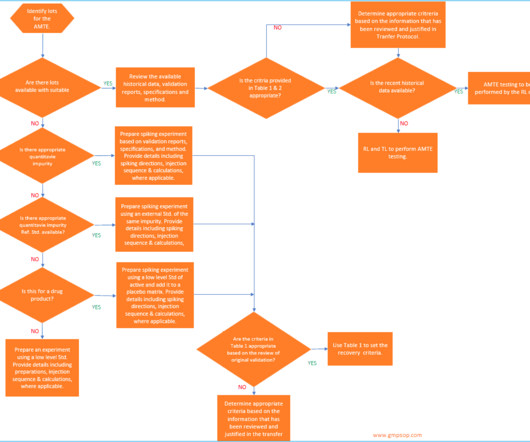
In general, you must formally validate all process steps, production equipment, cleaning methods, testing methods, computer systems and environment, facilities and utilities which are directly used for the manufacture of sterile and non-sterile products. This validation is relatively less comprehensive.








Let's personalize your content