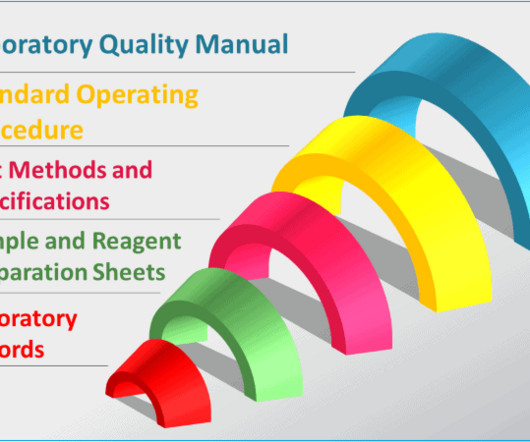
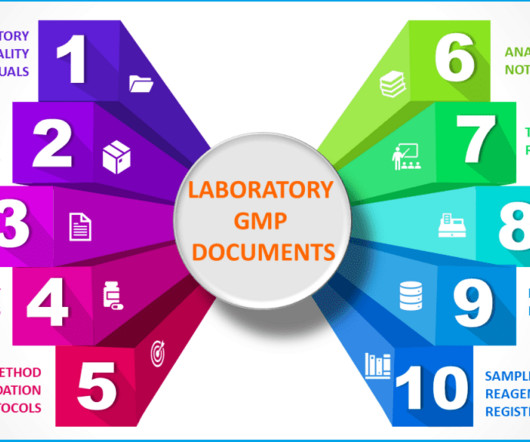
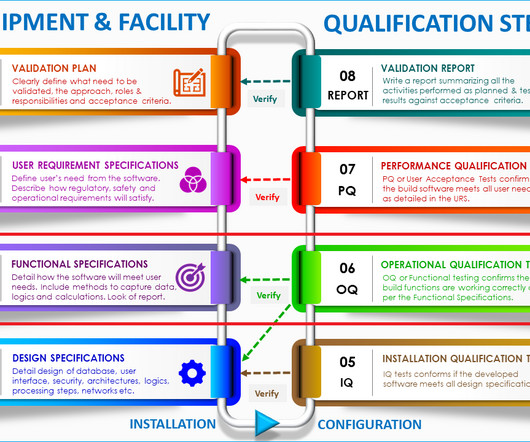
Typical GMP documentation in a quality control laboratory
GMPSOP
APRIL 3, 2023
In other word, you need GMP documentation specially developed and used for your laboratory to guide you through everything you do in the lab. Depending on the objective of the lab, the need for GMP documentation will change for the laboratory. The significance of well-designed GMP documentations is immense.









Let's personalize your content