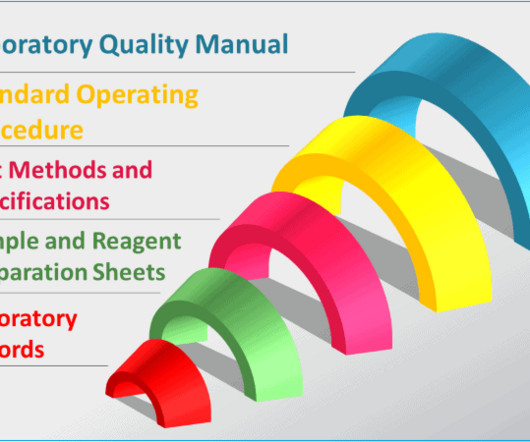
Typical GMP documentation in a quality control laboratory
GMPSOP
APRIL 3, 2023
There are also forms, logs, and registers to keep track of all the little details, as well as product specifications, analytical methods, manufacturing formulae, calculation of raw data and more. Sometime these are labelled as the “hidden factory”. Adapted and non-standard methods must be validated.









Let's personalize your content