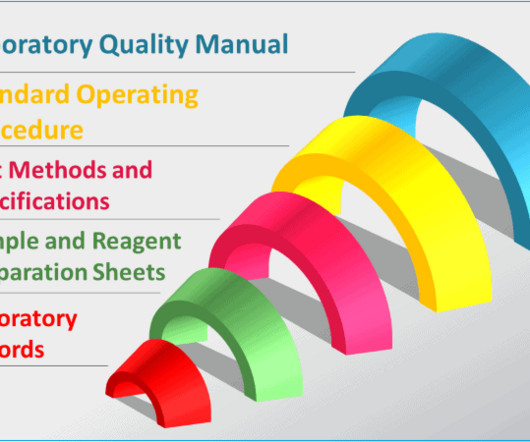
What are the elements of quality control process in pharmaceuticals
GMPSOP
OCTOBER 28, 2023
The analyst should ensure that the supervisor is notified as soon as an OOS event occurs, the sample is retained, and a documented investigation is commenced. Dealing with out-of-specification results Laboratory result out-of-specification events are a common occurrence in the quality control process.










Let's personalize your content