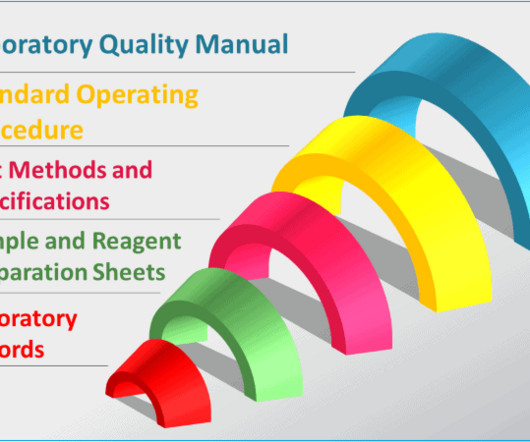
Typical GMP documentation in a quality control laboratory
GMPSOP
APRIL 3, 2023
To confirm your test results are trustworthy and unbiased, you would turn to well-designed policies, procedures, guidelines, methods, protocols and all types of records. In other word, you need GMP documentation specially developed and used for your laboratory to guide you through everything you do in the lab.









Let's personalize your content