Overview of GLP requirements on everyday laboratory operations
GMPSOP
DECEMBER 24, 2023
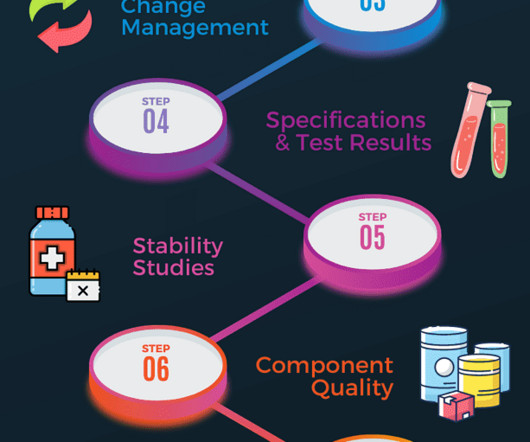
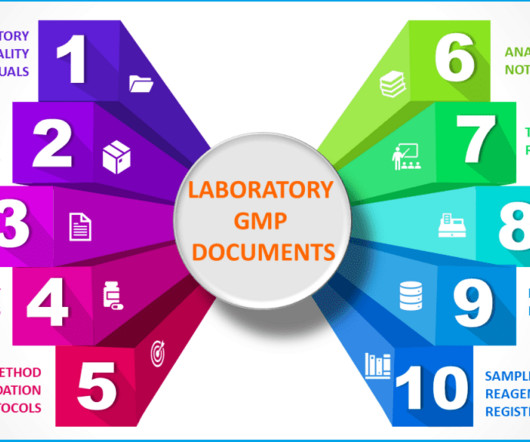
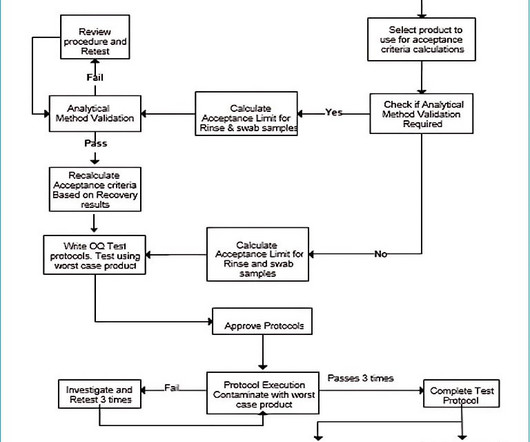
Additional documents included each month. The points below outline some areas in the codes where G(C)LP regulations are embedded in the GMP regulations and guidance documents. Additional documents included each month. All sampling procedures and plans must be documented. All written and updated by GMP experts.









Let's personalize your content