How to conduct product quality review in pharmaceutical
GMPSOP
NOVEMBER 19, 2023
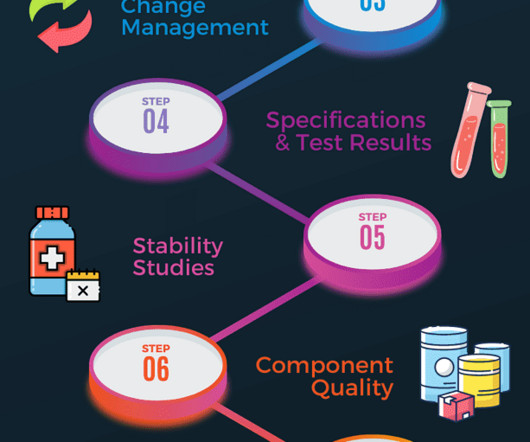
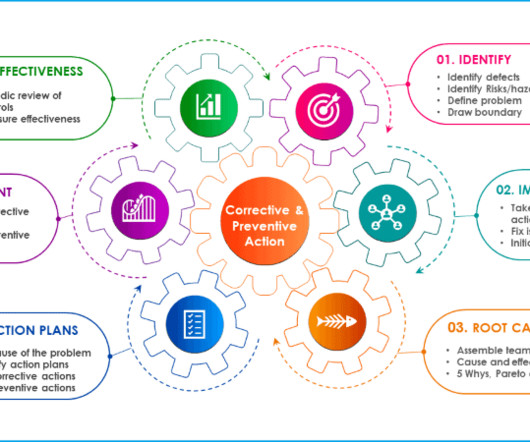
Additional documents included each month. Trending will help identify product and process improvements. If significant trends are identified, they should be documented, and changes should be made to avoid out-of-specification results. The number of batches manufactured, released and rejected should be documented.







Let's personalize your content