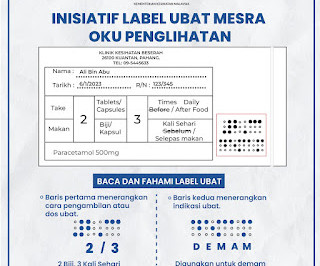
AYUSH, PHD Chamber of Commerce and Industry organise conference on labelling and packaging provisions for ASU products
Express Pharma
AUGUST 9, 2024
AYUSH Committee, PHD Chamber of Commerce and Industry organised a Conference on Labelling and Packaging Provisions for ASU products recently at PHD House, New Delhi. He also highlighted that a draft notification will soon be coming for the Industry on the indication of packaging.








































Let's personalize your content